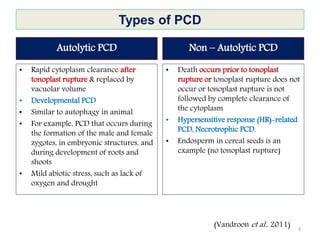
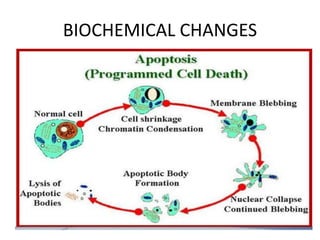
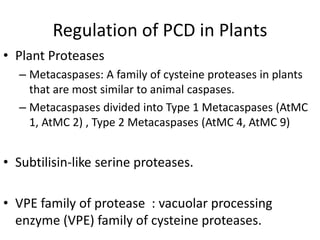
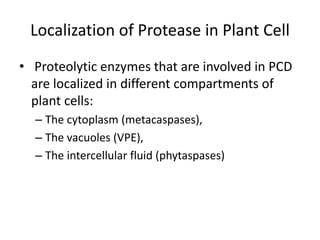
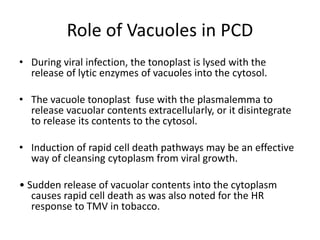
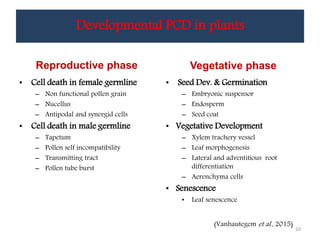
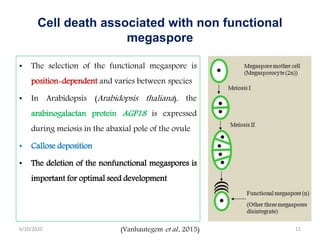
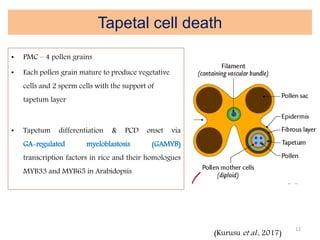
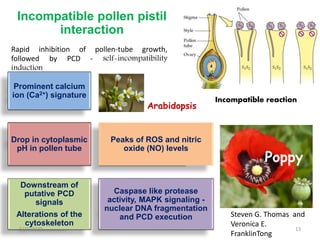
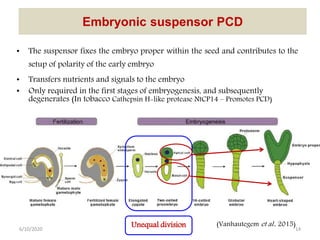
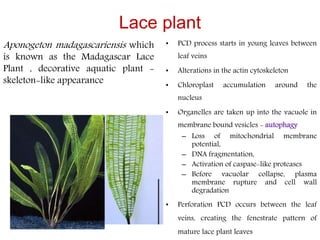
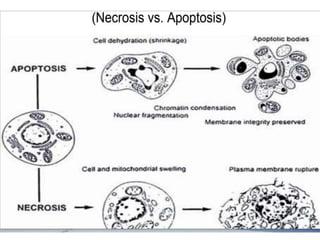
Programmed cell death (PCD) is an important physiological process in plants that involves the selective elimination of unwanted tissues through controlled cell destruction. There are two main types of PCD in plants - autolytic PCD, which involves rapid cytoplasm clearance after vacuole rupture, and non-autolytic PCD where death occurs prior to vacuole rupture. PCD plays essential roles in plant development and defense. The purpose of developmental PCD is to regulate cell division and shape tissues and organs. Defensive PCD helps control invading microbes. Biochemical changes involved in PCD regulation include the action of various proteases and the vacuole. PCD occurs in many developmental processes including reproduction, seed and root development, and senescence.