The generation of a stable master cell line
•
0 likes•17 views
The generation of a stable master cell line requires the integration of the expression cassette into the host cell genome. https://www.creativebiolabs.net/magic-mammalian-cell-expression-service.htm
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
More Related Content
What's hot
What's hot (16)
High yield production of therapeutic proteins in chloroplast

High yield production of therapeutic proteins in chloroplast
Protein expression and purification services from creative biomart

Protein expression and purification services from creative biomart
Strain improvement studies on L-aspaginase producing bacteria

Strain improvement studies on L-aspaginase producing bacteria
Strain development techniques of industrially important microorganisms

Strain development techniques of industrially important microorganisms
Bacterial efflux pump inhibitory activity of some indigenous medicinal plants...

Bacterial efflux pump inhibitory activity of some indigenous medicinal plants...
Similar to The generation of a stable master cell line
Similar to The generation of a stable master cell line (20)
Improved vector design eases cell line development workflow in the CHOZN GS-/...

Improved vector design eases cell line development workflow in the CHOZN GS-/...
Reducing time to clinic with CHOvolution™ cell line 

Reducing time to clinic with CHOvolution™ cell line
Scalability of Cell Culture Processes in Single-use Bioreactors using Differe...

Scalability of Cell Culture Processes in Single-use Bioreactors using Differe...
Muthamilselvan et al-2015-plant_biotechnology_journal

Muthamilselvan et al-2015-plant_biotechnology_journal
Molecular characterisation, attenuation and inactivation of very virulent inf...

Molecular characterisation, attenuation and inactivation of very virulent inf...
Recombinant Antibody Production: Current Methods and a Novel Antibody Generat...

Recombinant Antibody Production: Current Methods and a Novel Antibody Generat...
Platform Technologies to Accelerate Novel Vaccine Development and Manufacturing

Platform Technologies to Accelerate Novel Vaccine Development and Manufacturing
Platform Technologies to Accelerate Novel Vaccine Development and Manufacturing

Platform Technologies to Accelerate Novel Vaccine Development and Manufacturing
Recombinant Antibody Overview II - Creative Biolabs

Recombinant Antibody Overview II - Creative Biolabs
Thomas Sterovsky - Polymun Scientific (Vienna Conference, April 2016)

Thomas Sterovsky - Polymun Scientific (Vienna Conference, April 2016)
Exploring Intensified Seed Train Through Advancements in Perfusion Processing...

Exploring Intensified Seed Train Through Advancements in Perfusion Processing...
OS18 - 9.b.6 The use of reverse genetics to facilitate the growth of FMDV fo...

OS18 - 9.b.6 The use of reverse genetics to facilitate the growth of FMDV fo...
More from EchoHan4
More from EchoHan4 (20)
Recently uploaded
Recently uploaded (20)
MSC IV_Forensic medicine - Mechanical injuries.pdf

MSC IV_Forensic medicine - Mechanical injuries.pdf
Nanoparticles for the Treatment of Alzheimer’s Disease_102718.pptx

Nanoparticles for the Treatment of Alzheimer’s Disease_102718.pptx
SaffronCrocusGenomicsThessalonikiOnlineMay2024TalkOnline.pptx

SaffronCrocusGenomicsThessalonikiOnlineMay2024TalkOnline.pptx
Soil and Water Conservation Engineering (SWCE) is a specialized field of stud...

Soil and Water Conservation Engineering (SWCE) is a specialized field of stud...
Factor Causing low production and physiology of mamary Gland

Factor Causing low production and physiology of mamary Gland
Mining Activity and Investment Opportunity in Myanmar.pptx

Mining Activity and Investment Opportunity in Myanmar.pptx
POST TRANSCRIPTIONAL GENE SILENCING-AN INTRODUCTION.pptx

POST TRANSCRIPTIONAL GENE SILENCING-AN INTRODUCTION.pptx
Heat Units in plant physiology and the importance of Growing Degree days

Heat Units in plant physiology and the importance of Growing Degree days
The generation of a stable master cell line
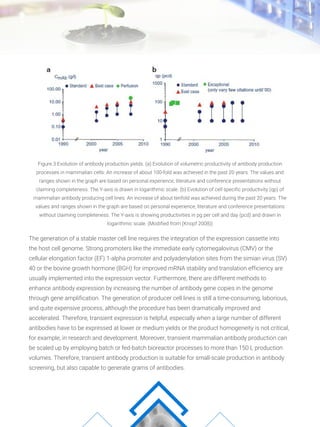
- 1. The generation of a stable master cell line requires the integration of the expression cassette into the host cell genome. Strong promoters like the immediate early cytomegalovirus (CMV) or the cellular elongation factor (EF) 1-alpha promoter and polyadenylation sites from the simian virus (SV) 40 or the bovine growth hormone (BGH) for improved mRNA stability and translation efficiency are usually implemented into the expression vector. Furthermore, there are different methods to enhance antibody expression by increasing the number of antibody gene copies in the genome through gene amplification. The generation of producer cell lines is still a time-consuming, laborious, and quite expensive process, although the procedure has been dramatically improved and accelerated. Therefore, transient expression is helpful, especially when a large number of different antibodies have to be expressed at lower or medium yields or the product homogeneity is not critical, for example, in research and development. Moreover, transient mammalian antibody production can be scaled up by employing batch or fed-batch bioreactor processes to more than 150 L production volumes. Therefore, transient antibody production is suitable for small-scale production in antibody screening, but also capable to generate grams of antibodies. Figure.3 Evolution of antibody production yields. (a) Evolution of volumetric productivity of antibody production processes in mammalian cells. An increase of about 100-fold was achieved in the past 20 years. The values and ranges shown in the graph are based on personal experience, literature and conference presentations without claiming completeness. The Y-axis is drawn in logarithmic scale. (b) Evolution of cell specific productivity (qp) of mammalian antibody producing cell lines. An increase of about tenfold was achieved during the past 20 years. The values and ranges shown in the graph are based on personal experience, literature and conference presentations without claiming completeness. The Y-axis is showing productivities in pg per cell and day (pcd) and drawn in logarithmic scale. (Modified from (Knopf 2008))