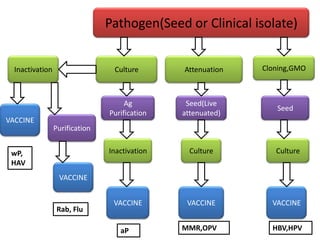
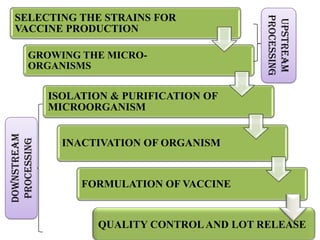
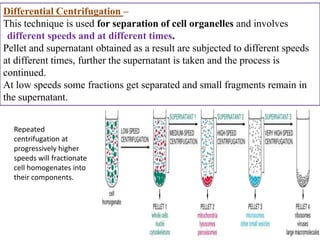
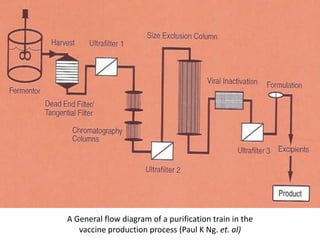
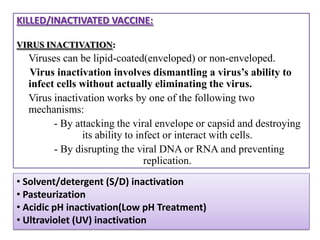
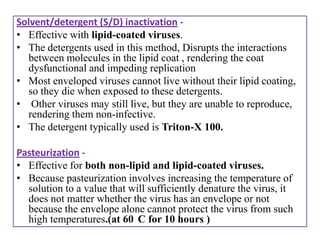
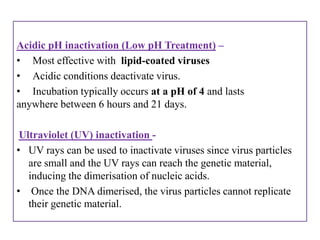
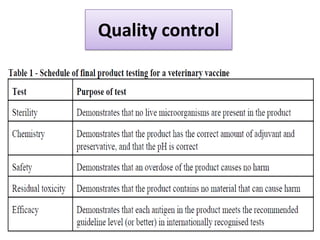
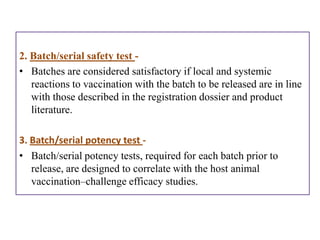
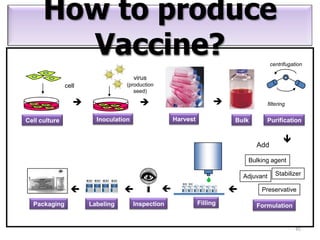
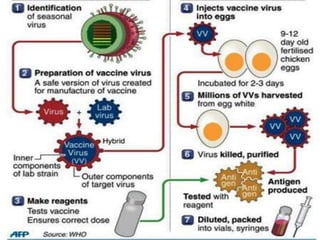
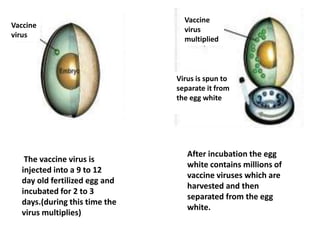
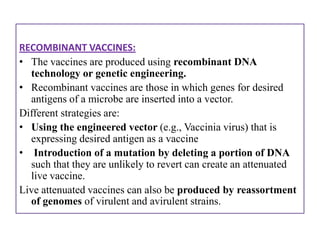
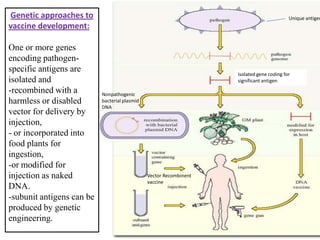
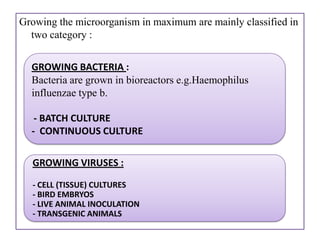
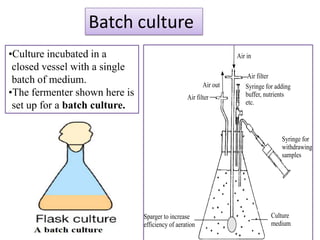
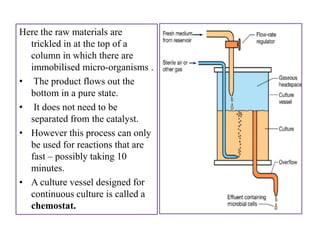
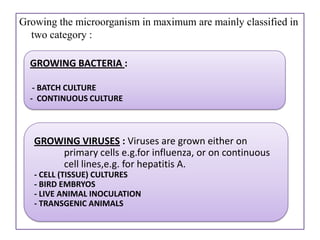
The document outlines various techniques for vaccine production, including the use of live attenuated and inactivated vaccines, as well as modern gene-based methods for antigen production. It describes the processes involved in growing microorganisms, isolating and purifying the desired antigens, and inactivating viruses for safety. Quality control measures ensure vaccine purity, safety, potency, and compliance with production standards before release to the market.