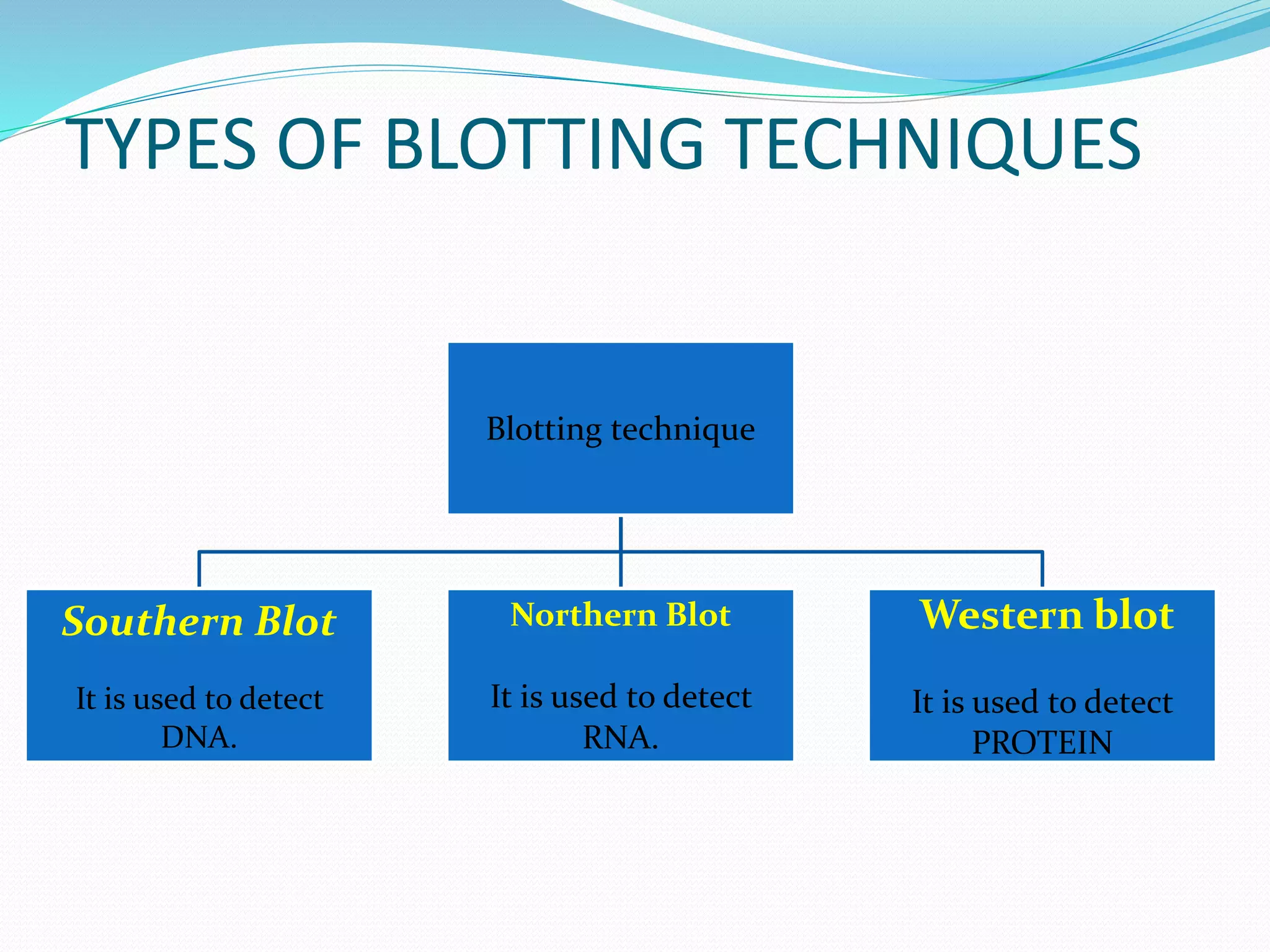
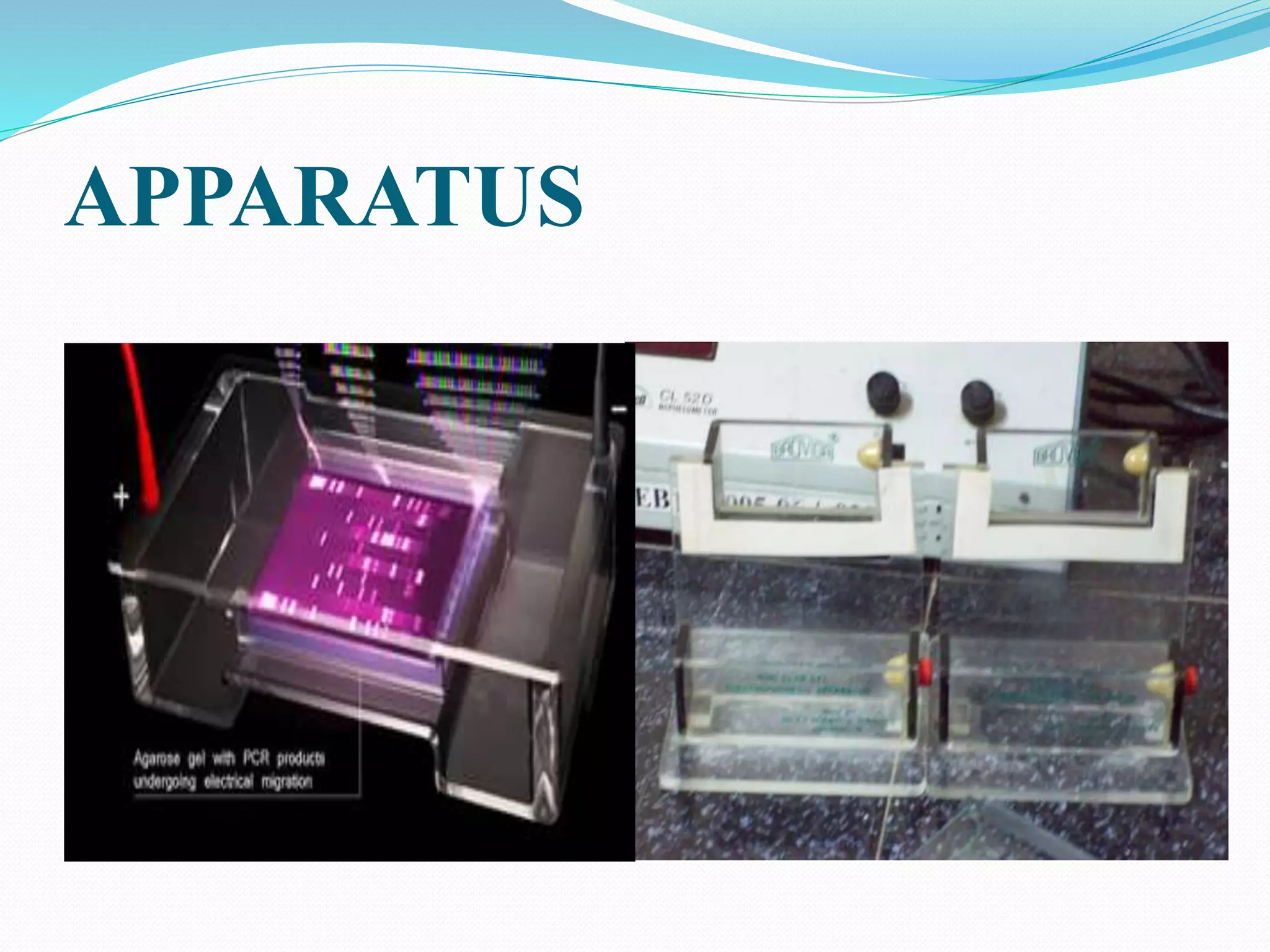
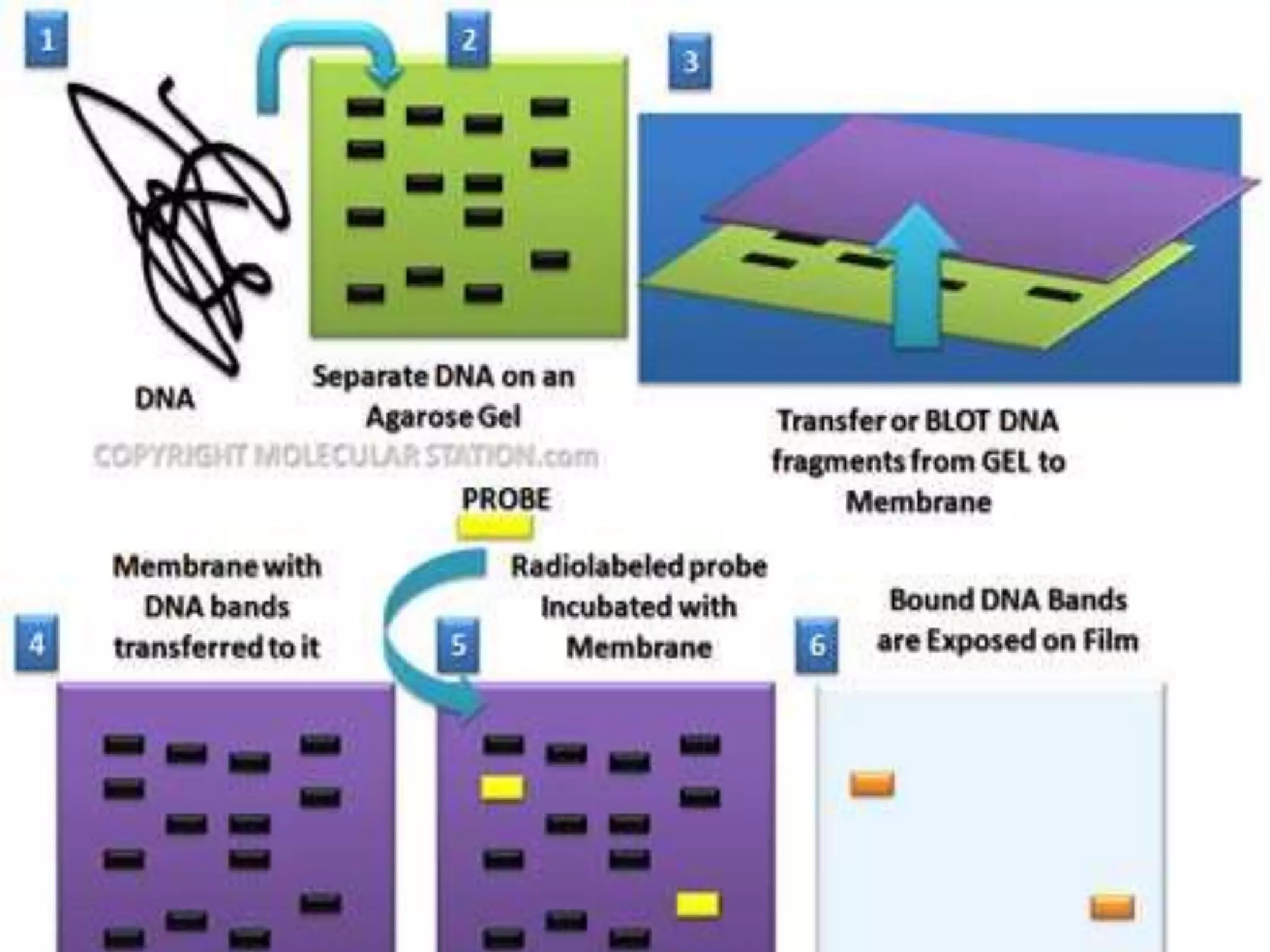
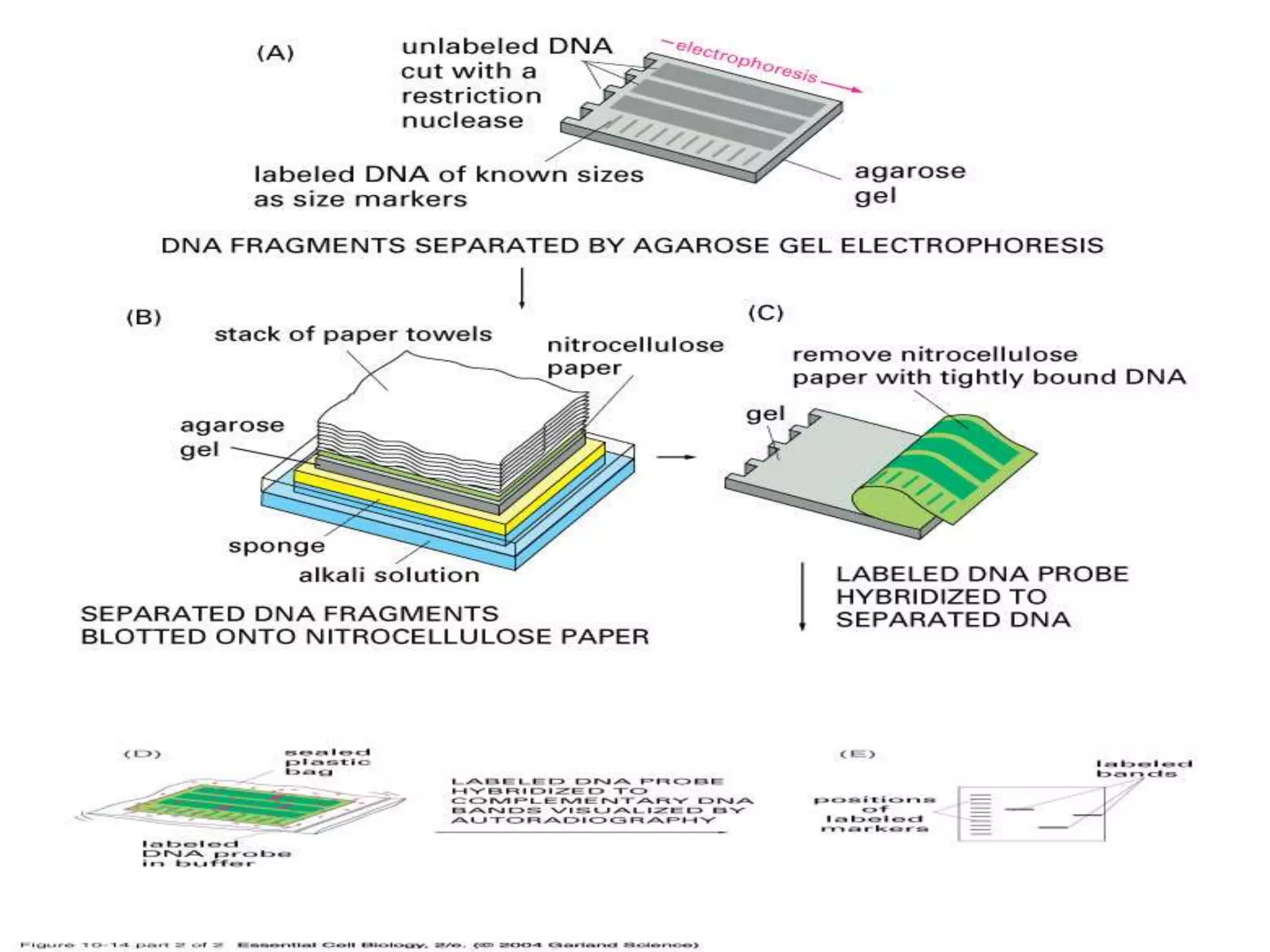
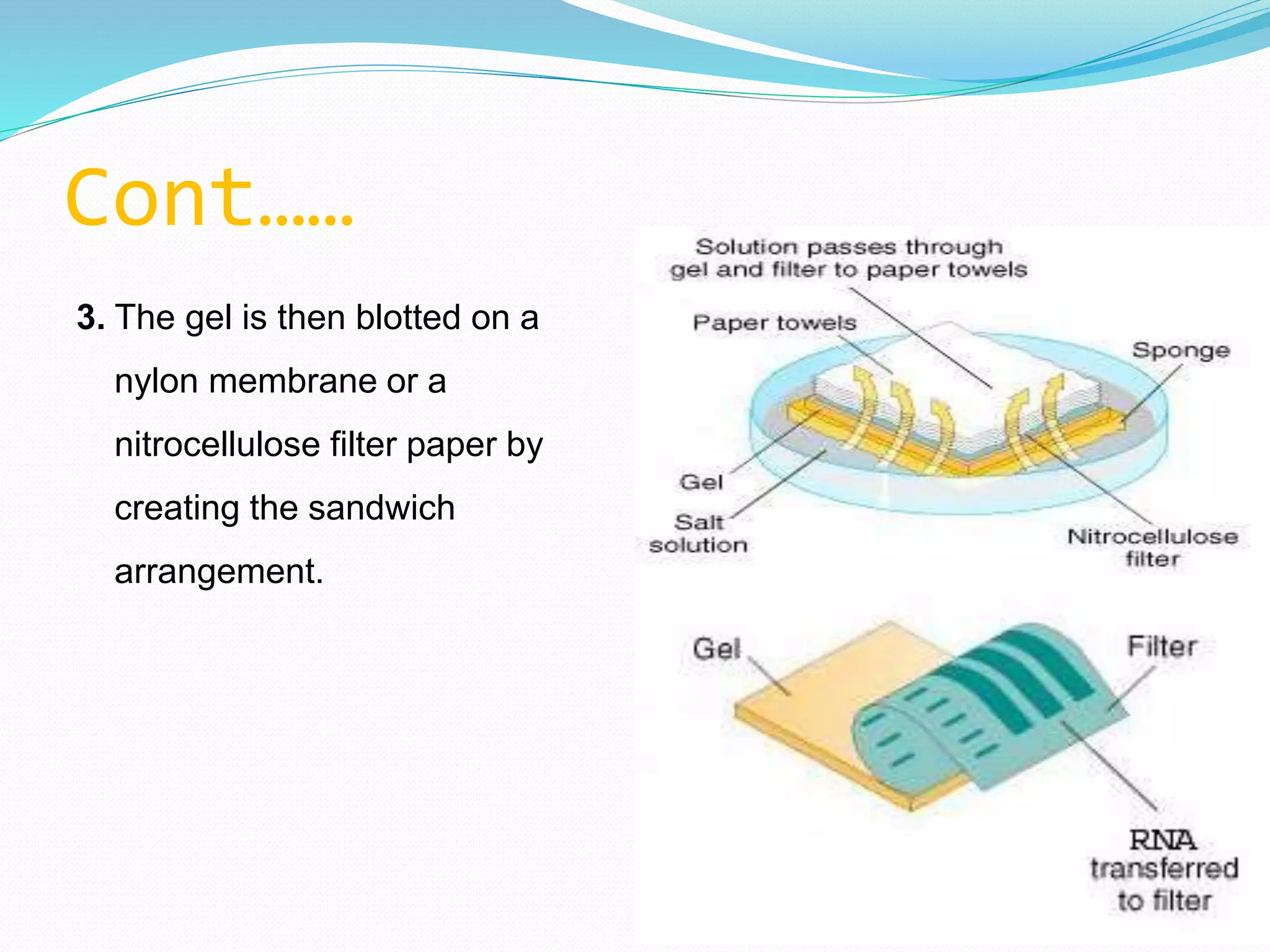
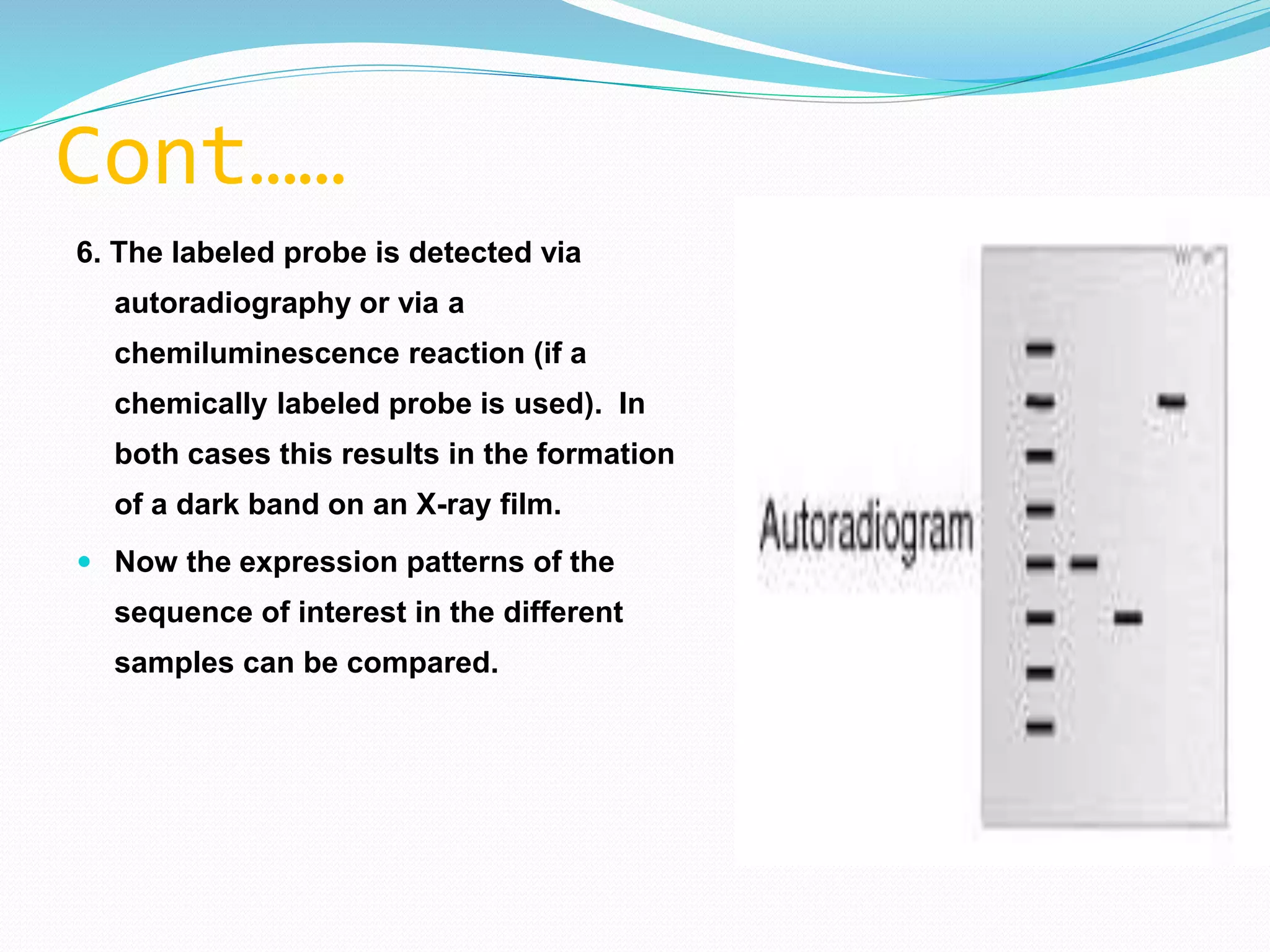
This document discusses various blotting techniques used to detect and analyze biomolecules like DNA, RNA, and proteins. It describes the Southern blot technique developed by Edwin Southern used to detect specific DNA sequences. It also discusses the Northern blot technique used to detect RNA, developed by James Alwine and George Stark. Finally, it summarizes the Western blot technique used to detect specific proteins by using antibodies, developed in 1981. These blotting techniques allow separation and detection of biomolecules through transfer and hybridization/binding reactions.