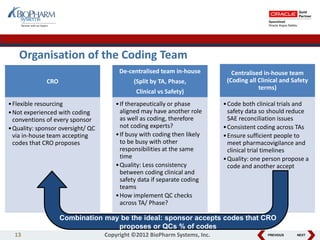
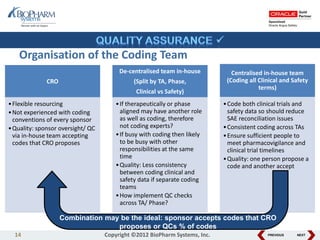
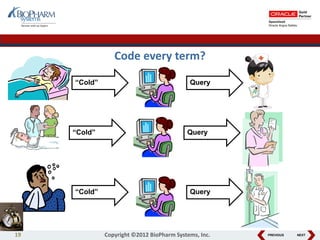
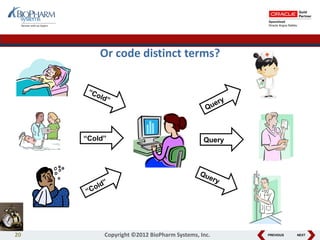
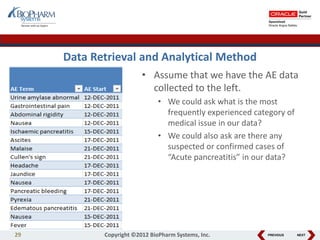
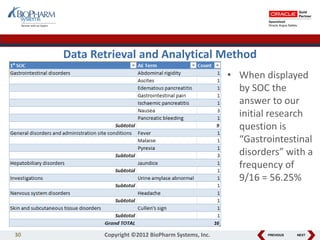
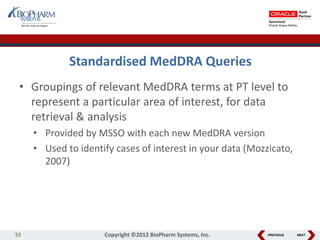
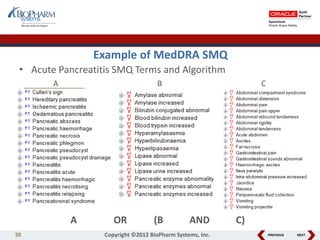
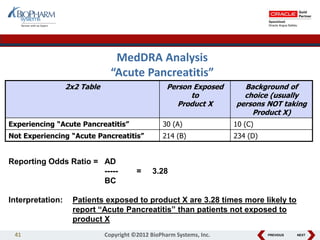
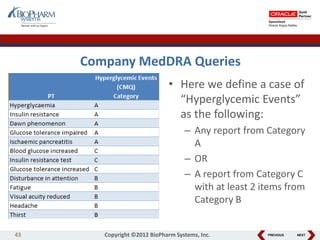
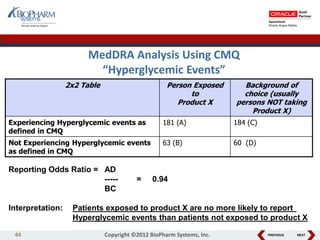
This document discusses best practices for medical coding with MedDRA. It covers issues and decisions around coding practice, such as who should code reports, which terms to code, ensuring coding quality, and optimizing technology. It also discusses issues and decisions around data analysis, such as using standardized MedDRA queries and medical dictionary hierarchies for data retrieval and grouping terms to answer research questions. The presentation aims to provide guidance on medical coding processes and analyses.