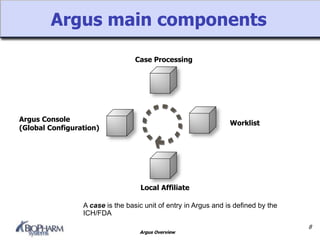
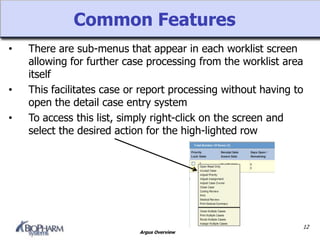
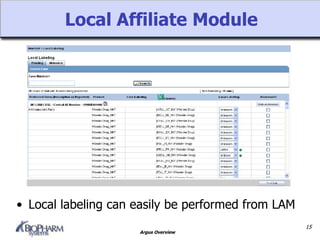
Argus is a pharmacovigilance software that supports global safety management through flexible configuration, integrated workflow and reporting features. It allows cases to be entered locally and sent to a central repository for review. Argus facilitates global case processing and labeling through worklists and notification tools. Reports can be generated in local languages to aid review and regulatory submission tracking in different countries.