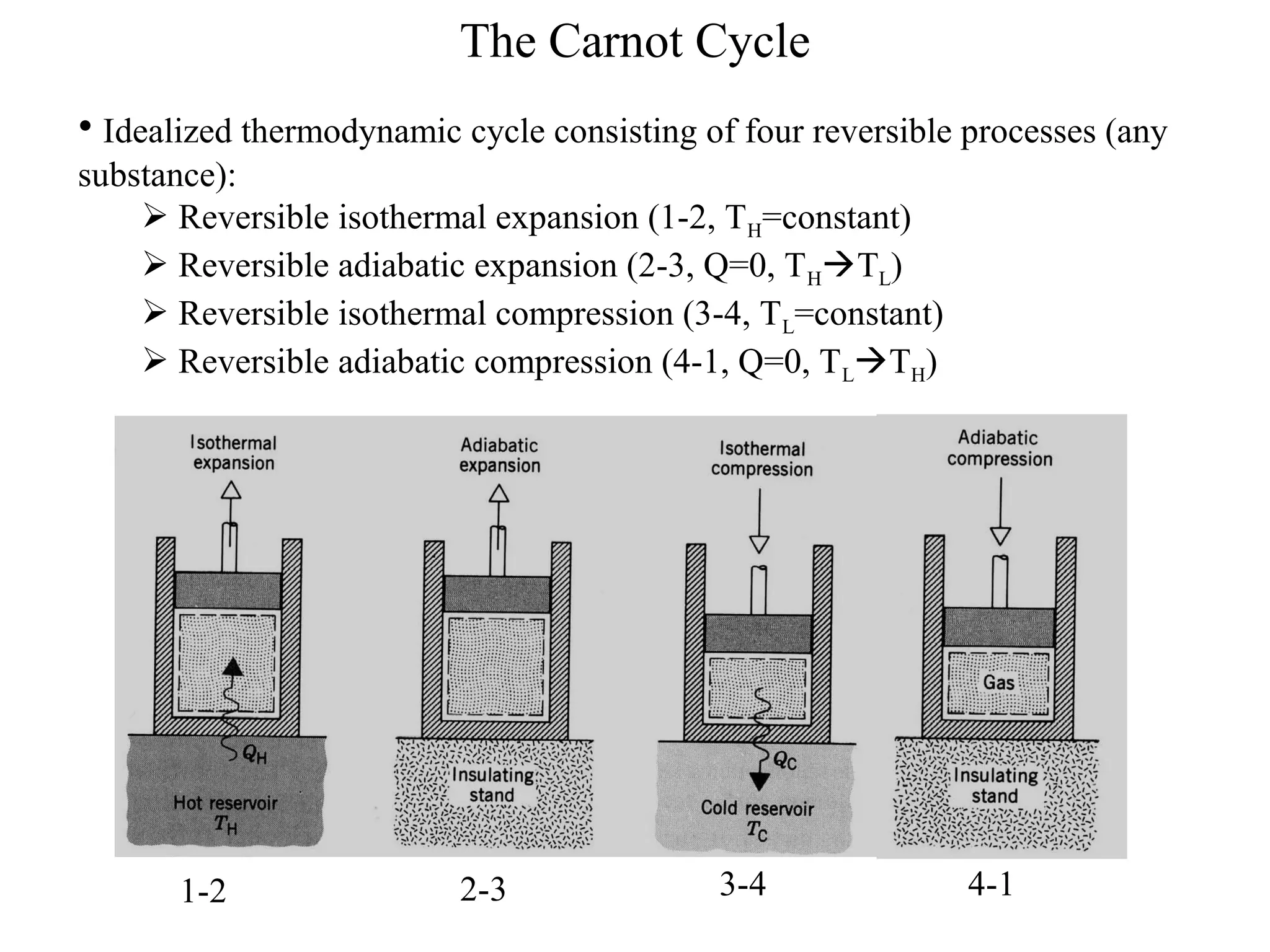
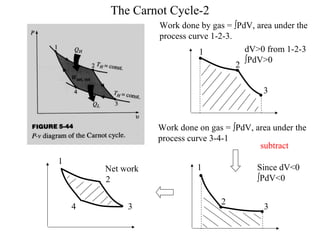
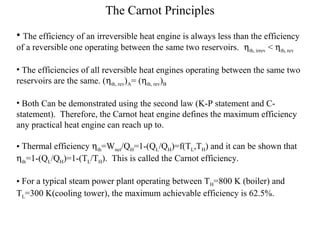
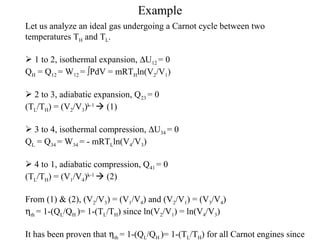
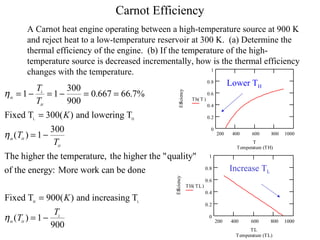
The Carnot Cycle describes an ideal thermodynamic cycle consisting of four reversible processes involving any substance: (1) reversible isothermal expansion, (2) reversible adiabatic expansion, (3) reversible isothermal compression, and (4) reversible adiabatic compression. The efficiency of the Carnot Cycle, known as the Carnot efficiency, is the maximum possible efficiency for any heat engine operating between two temperature reservoirs and is equal to 1 - (TL/TH), where TL and TH are the temperatures of the low and high reservoirs.