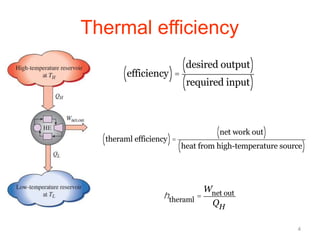
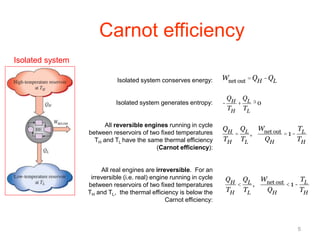
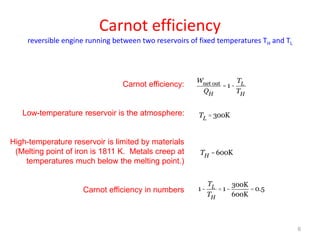
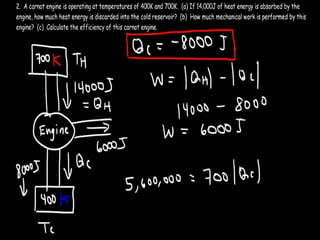
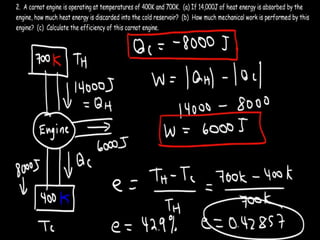
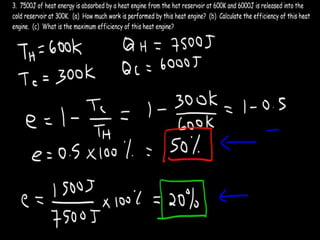
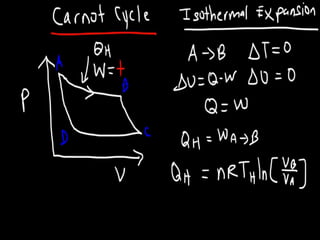
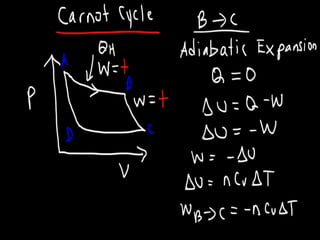
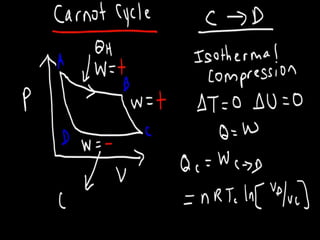
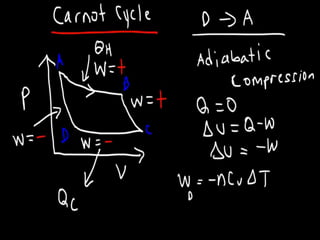
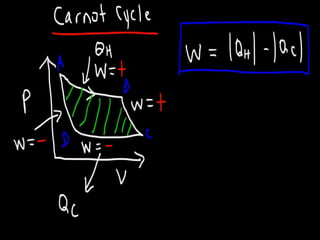
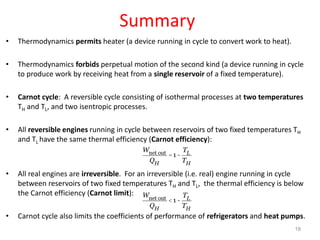
The document discusses the Carnot cycle. It begins by establishing the concept of entropy and analyzing the Carnot cycle as any other thermodynamic process. The Carnot cycle consists of isothermal processes at two temperatures TH and TL and two isentropic processes. All reversible engines running in a cycle between reservoirs at two fixed temperatures TH and TL have the same maximum thermal efficiency known as the Carnot efficiency. Real engines are irreversible and have a thermal efficiency below the Carnot limit.