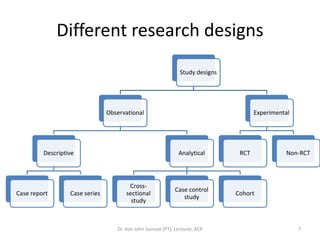
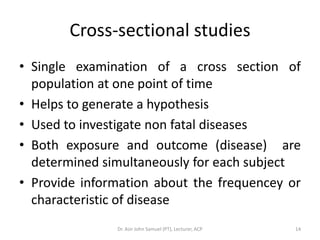
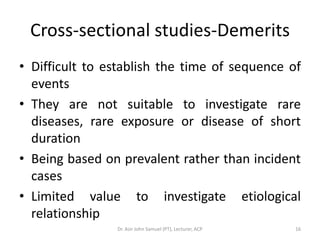
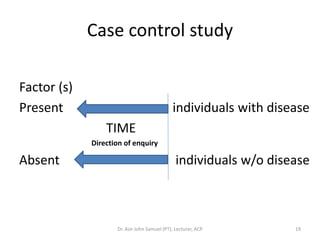
1. The document discusses various research designs including descriptive designs like case reports, case series, and cross-sectional studies as well as analytical designs like case-control and cohort studies.
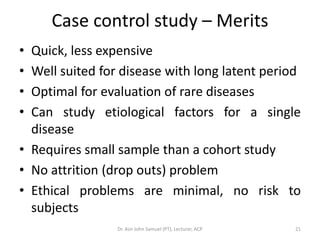
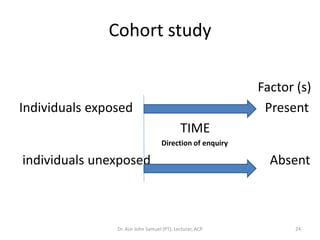
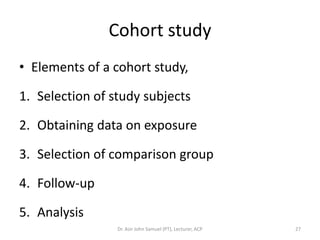
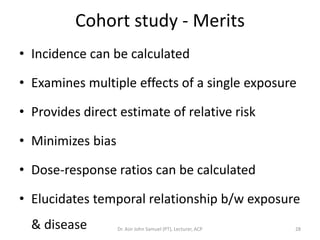
2. Key aspects of different research designs are explained, including their merits and limitations. For example, case reports are useful for rare diseases but cannot assess statistical associations, while cohort studies directly measure risk but are time-consuming.
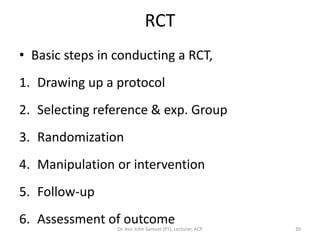
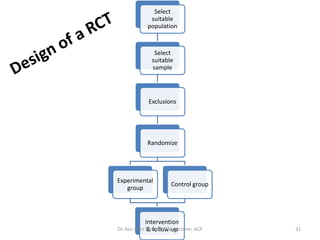
3. Randomized controlled trials are covered, outlining basic steps like drawing protocols, randomization, and intervention/follow-up. Randomization techniques like simple, block, and stratified methods are also summarized.