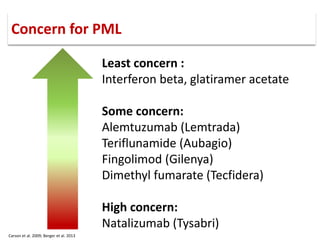
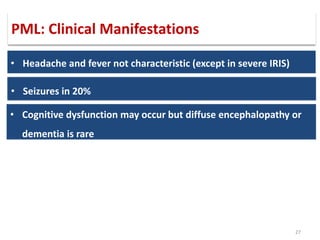
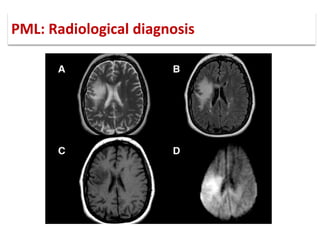
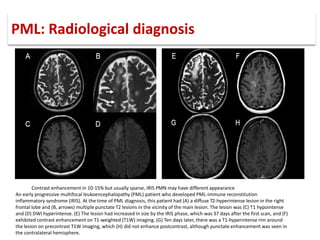
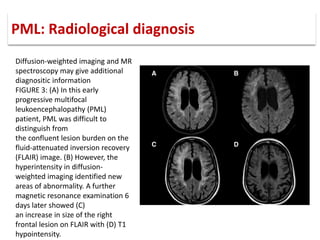
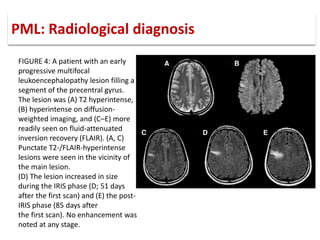
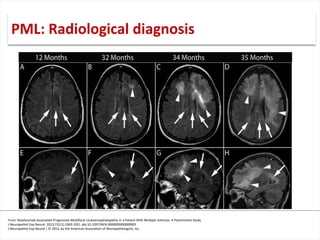
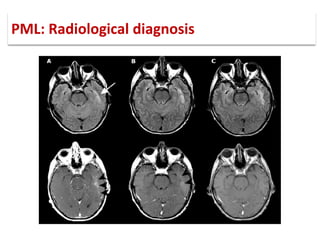
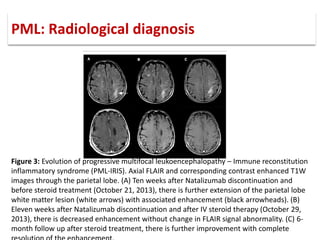
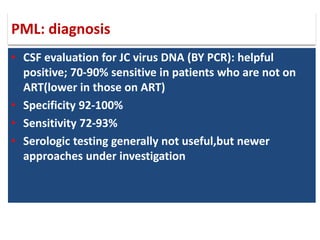
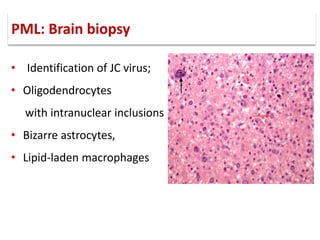
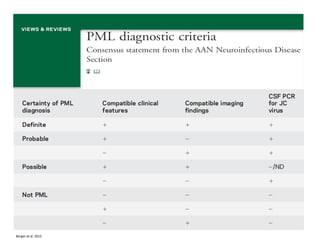
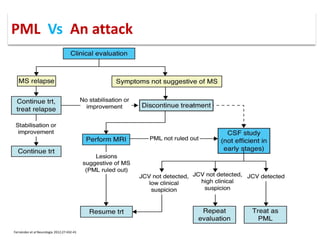
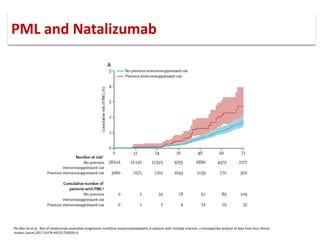
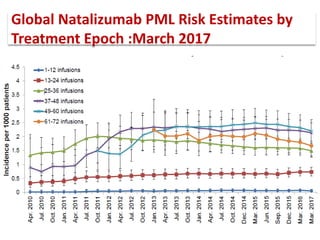
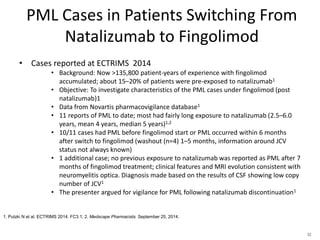
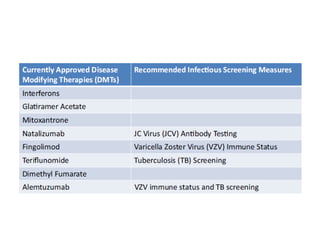
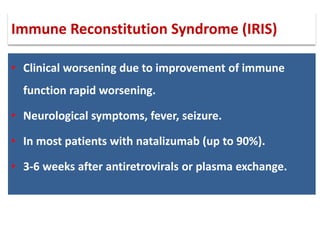
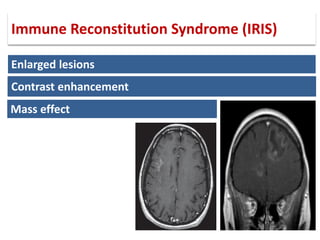
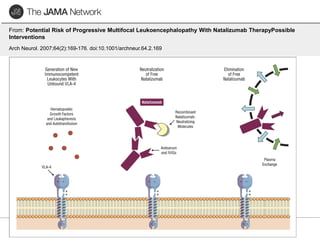
This document discusses progressive multifocal leukoencephalopathy (PML), an opportunistic infection caused by the John Cunningham virus (JCV) that leads to demyelination in the central nervous system. It highlights the epidemiology, clinical manifestations, diagnostic methods, and risk factors associated with PML, particularly in immunocompromised patients and those undergoing immunomodulatory therapies. It also presents data on the incidence of PML in patients treated with natalizumab and other therapies, addressing the relationships between these treatments and the development of PML.

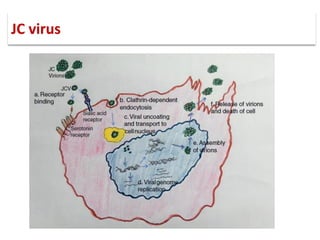
![▪ John Cunningham virus (JCV) is a
double-stranded DNA
polyomavirus.
▪ Takes its name from the initials of
the patient from whom it was
first isolated.
▪ PML was initially described in
patients with underlying B-cell
lymphoproliferative disorders [1]
JC virus](https://image.slidesharecdn.com/pml-220423195132/85/Progressive-multifocal-leukoencephalopathy-PML-2-320.jpg)

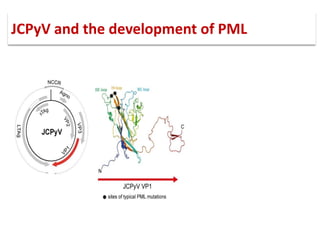

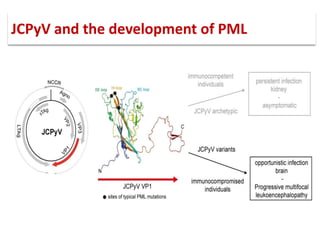

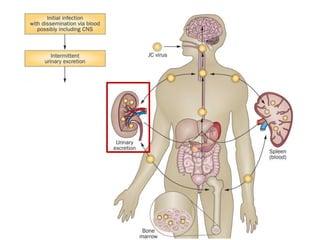

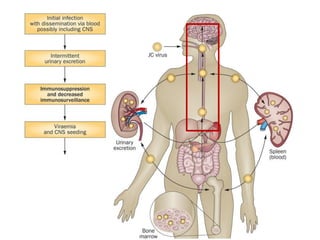
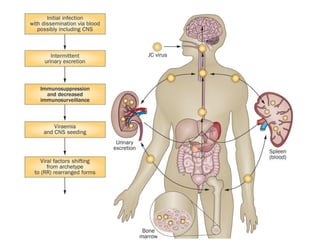
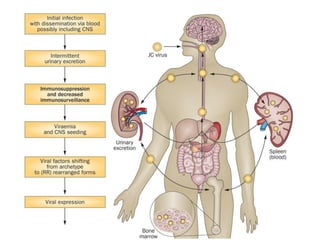
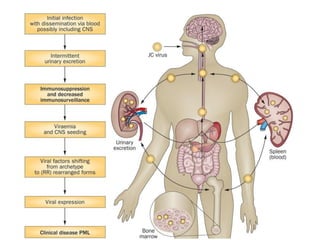
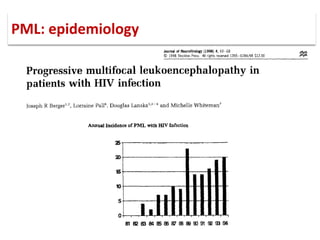
![▪ 50-90% of adults have been exposed to this virus
▪ 19-27% of these people shedding JCV in their urine.[5,7-10]
▪ Acquisition of this virus is not associated with a clinical
syndrome.[9]
▪ Opportunistic infection, caused by the polyoma virus JC virus
▪ Characterized by focal demyelination in the CNS
▪ Worldwide distribution, seroprevalence of 39-69% in adults
▪ Primary infection usually in childhood
▪ No recognized acute JC virus infection
▪ Likely asymptomatic chronic carrier state
PML: epidemiology](https://image.slidesharecdn.com/pml-220423195132/85/Progressive-multifocal-leukoencephalopathy-PML-15-320.jpg)