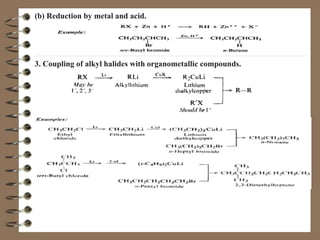
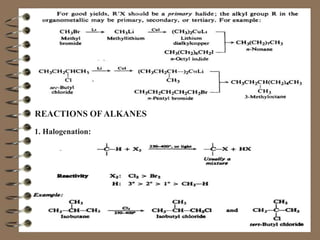
This document provides an overview of alkanes. It begins by defining hydrocarbons and explaining that aliphatic hydrocarbons are divided into families including alkanes. Methane, CH4, is introduced as the simplest alkane. The document then discusses the structure, naming conventions, reactions including halogenation and combustion, and preparation of alkanes. Physical properties such as being colorless, odorless, and nonpolar are also mentioned.