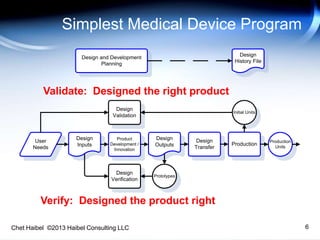
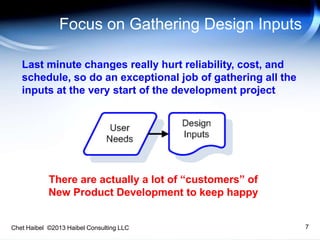
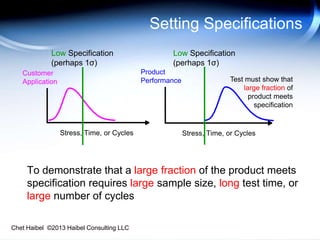
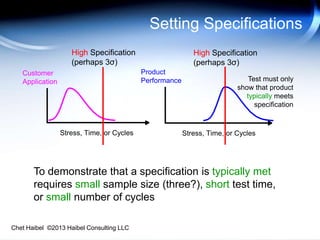
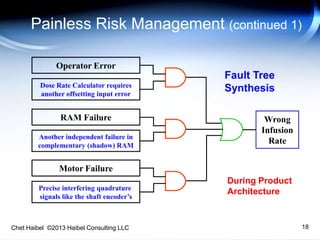
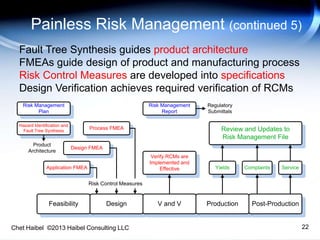
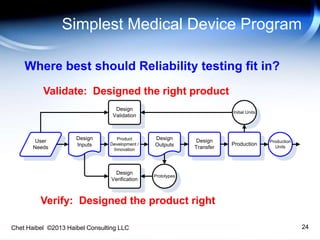
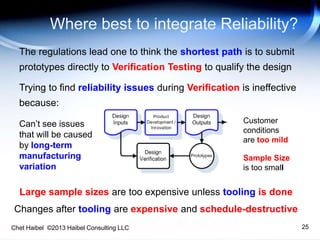
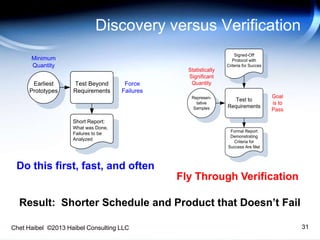
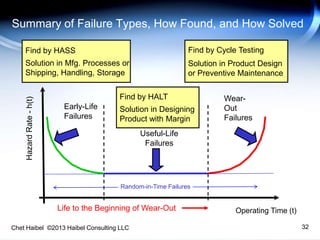
The document discusses strategies for improving the efficiency and reliability of medical devices through effective risk management and quality control processes. It emphasizes the importance of early identification of hazards, rigorous testing, and specification setting to enhance product reliability while complying with relevant regulations. The author details methodologies such as fault tree analysis and failure mode effects analysis to ensure comprehensive risk assessment throughout the product lifecycle.


















![Chet Haibel ©2013 Haibel Consulting LLC 33
Accelerated (Wear-Out) Test
If possible, set up a repetitive “cycle test” which removes the
“dead time” between cycles. But brainstorm what artifact the
test may be adding and / or what the test may be concealing
Test until a minimum of five failures are produced [Haibel’s rule]
Use Weibull Analysis to fit a distribution to the failure data
If life is not sufficient, determine the reservoir of material and
the process consuming the reservoir. Increase the reservoir
of material and / or slow down the process consuming it
If necessary, replace the reservoir of material periodically
with a scheduled preventive maintenance program](https://image.slidesharecdn.com/medicaldevicereliabilityprogram12may2011rev1-111205093052-phpapp02/85/Medical-device-reliability-program-33-320.jpg)

