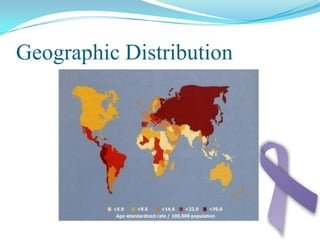
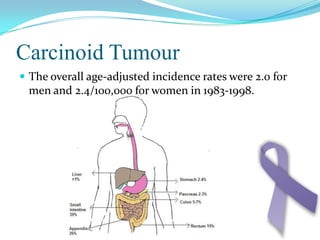
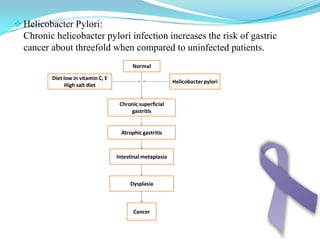
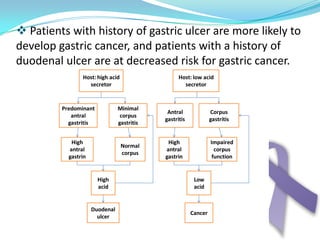
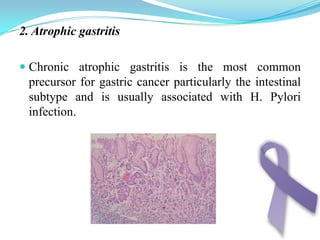
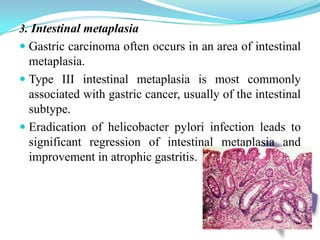
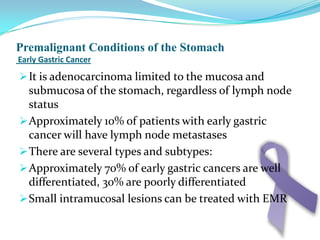
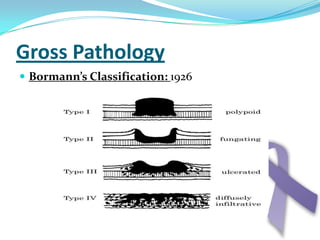
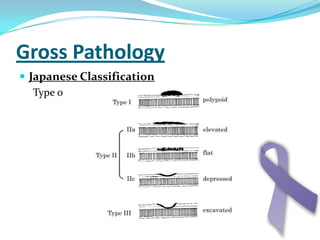
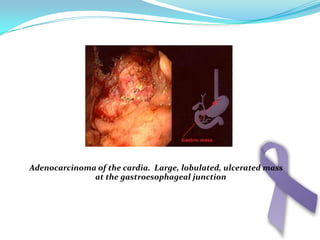
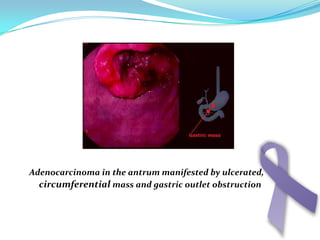
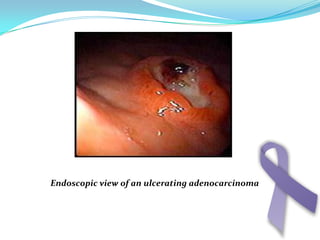
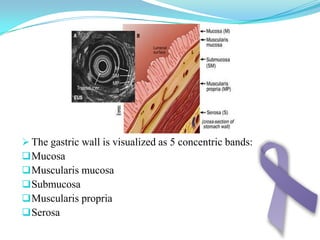
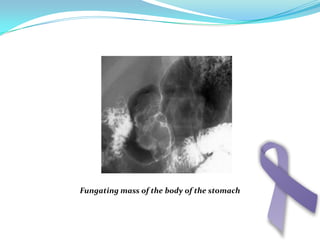
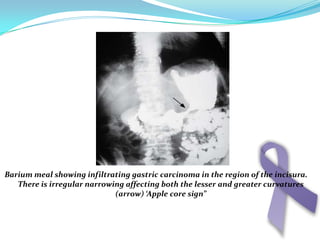
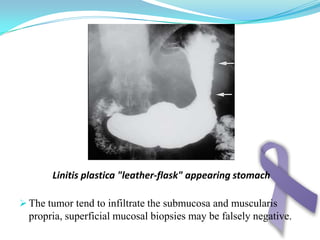
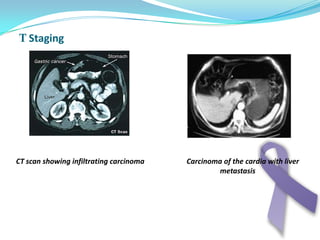
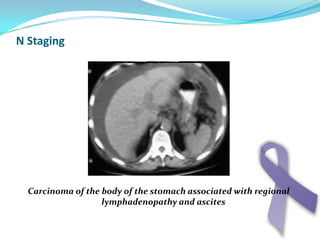
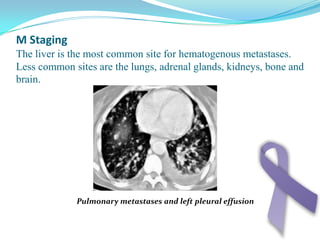
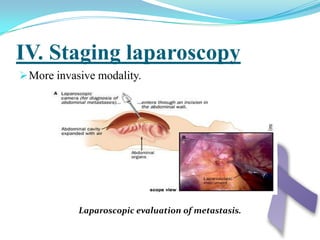
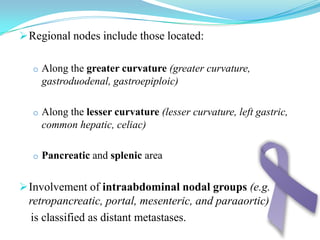
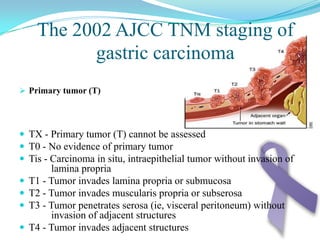
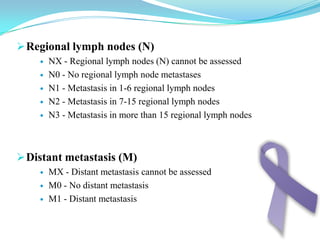
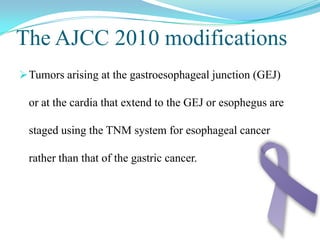
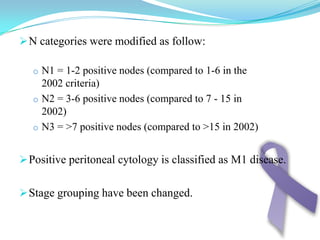
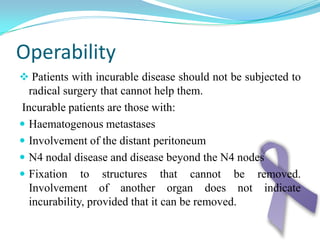
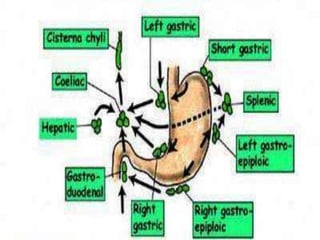
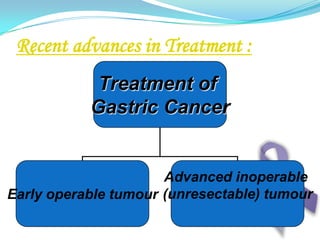
Gastric cancer is the second most common cancer worldwide. It is most common in elderly men over 65 years old. Risk factors include family history, diet high in salt/fat/nitrates, H. pylori infection, and atrophic gastritis. Premalignant conditions include polyps, intestinal metaplasia, and dysplasia. Symptoms include dyspepsia and pain induced by meat that does not respond to treatment. Staging systems include Bormann's classification and the Japanese classification. Histologically, Lauren's classification divides gastric cancer into intestinal and diffuse types based on cell morphology and growth pattern.