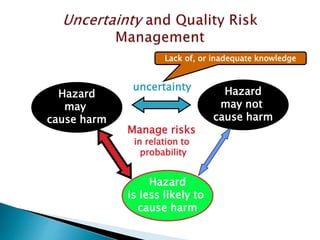
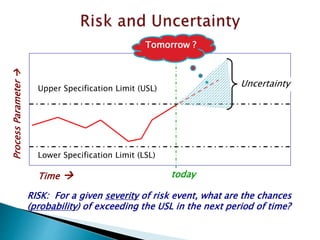
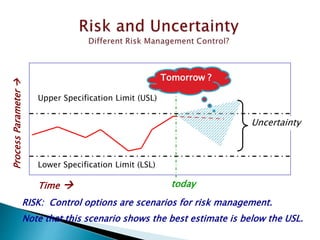
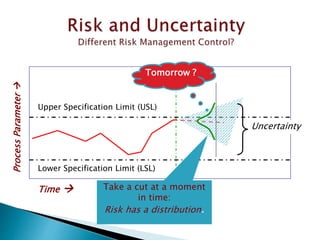
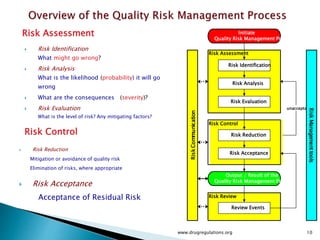
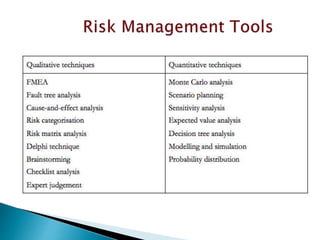
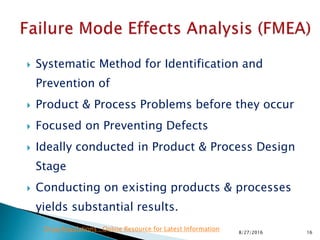
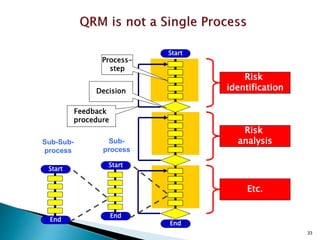
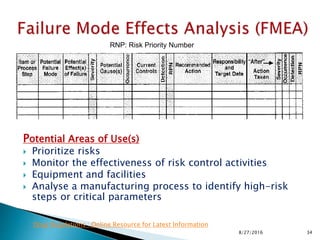
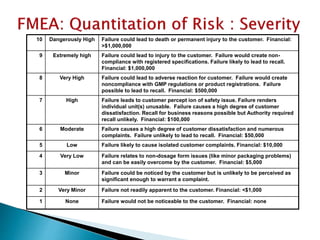
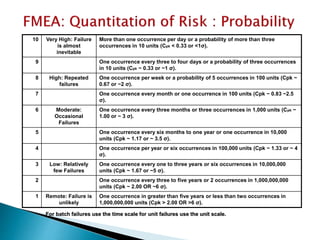
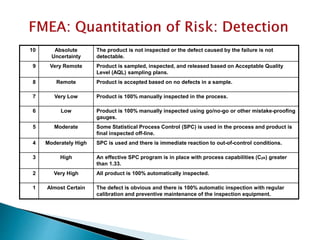
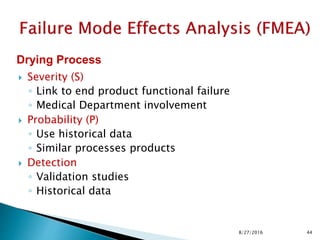
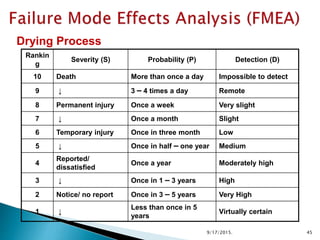
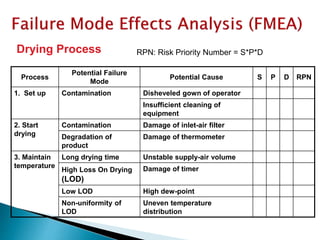
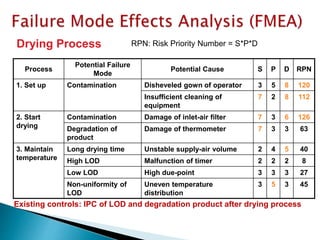
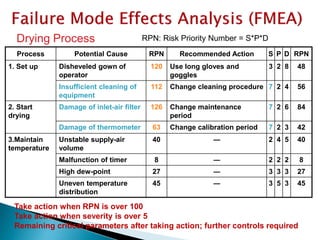
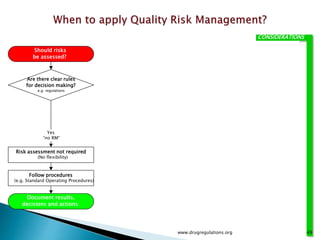
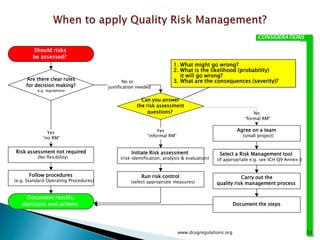
This document provides an overview of quality risk management and failure mode and effects analysis (FMEA). It discusses risk as a combination of the probability of harm occurring and the severity of that harm. The quality risk management process includes risk identification, analysis, evaluation, control, and communication. FMEA is presented as a systematic method to identify and prevent product and process problems before they occur. Key aspects of FMEA covered include failure modes, effects, risk priority numbers, and using FMEA to prioritize risks for improvement actions. Scales for rating severity, occurrence, detection, and examples of applying FMEA to a drying process are also presented.