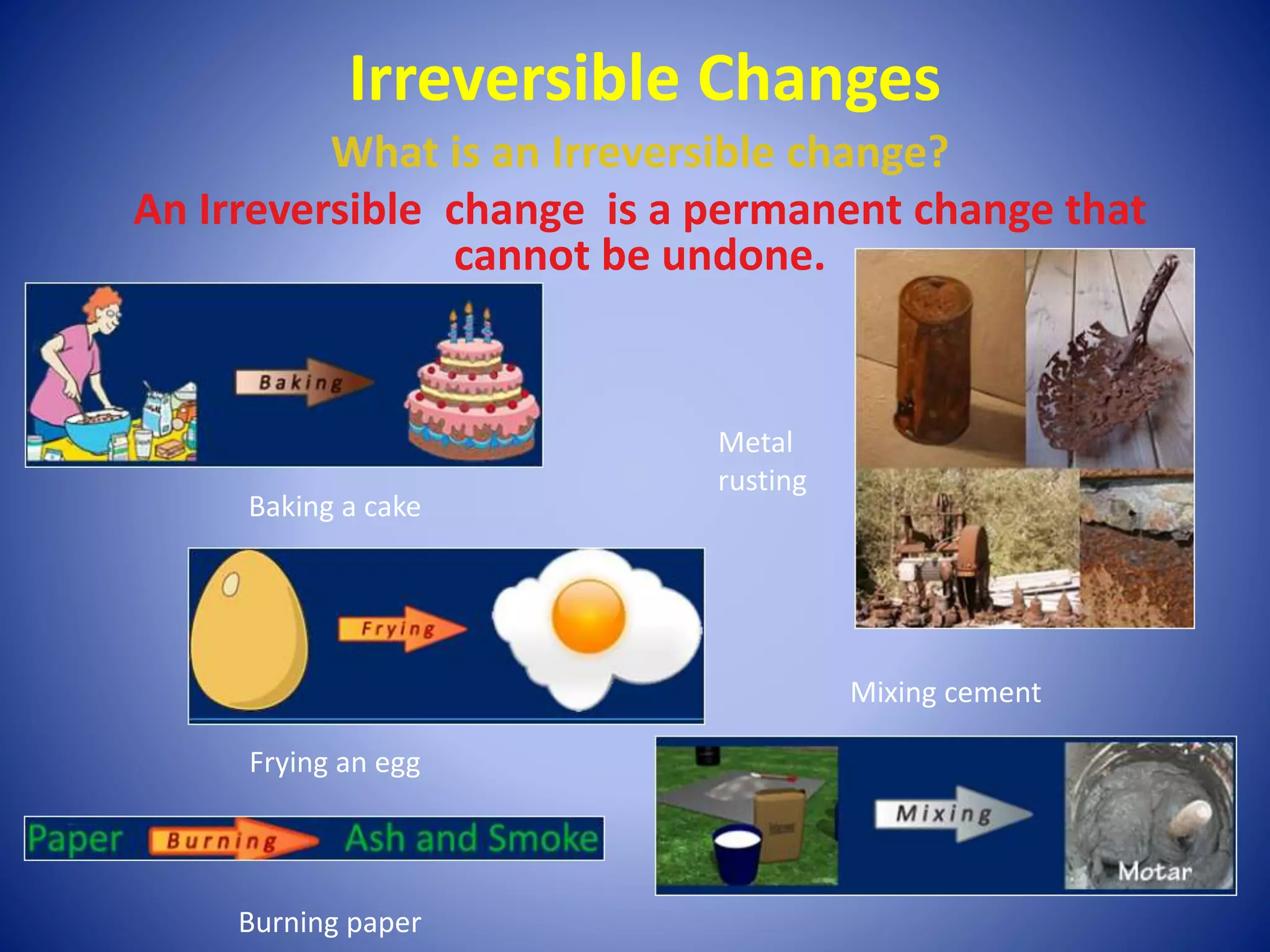
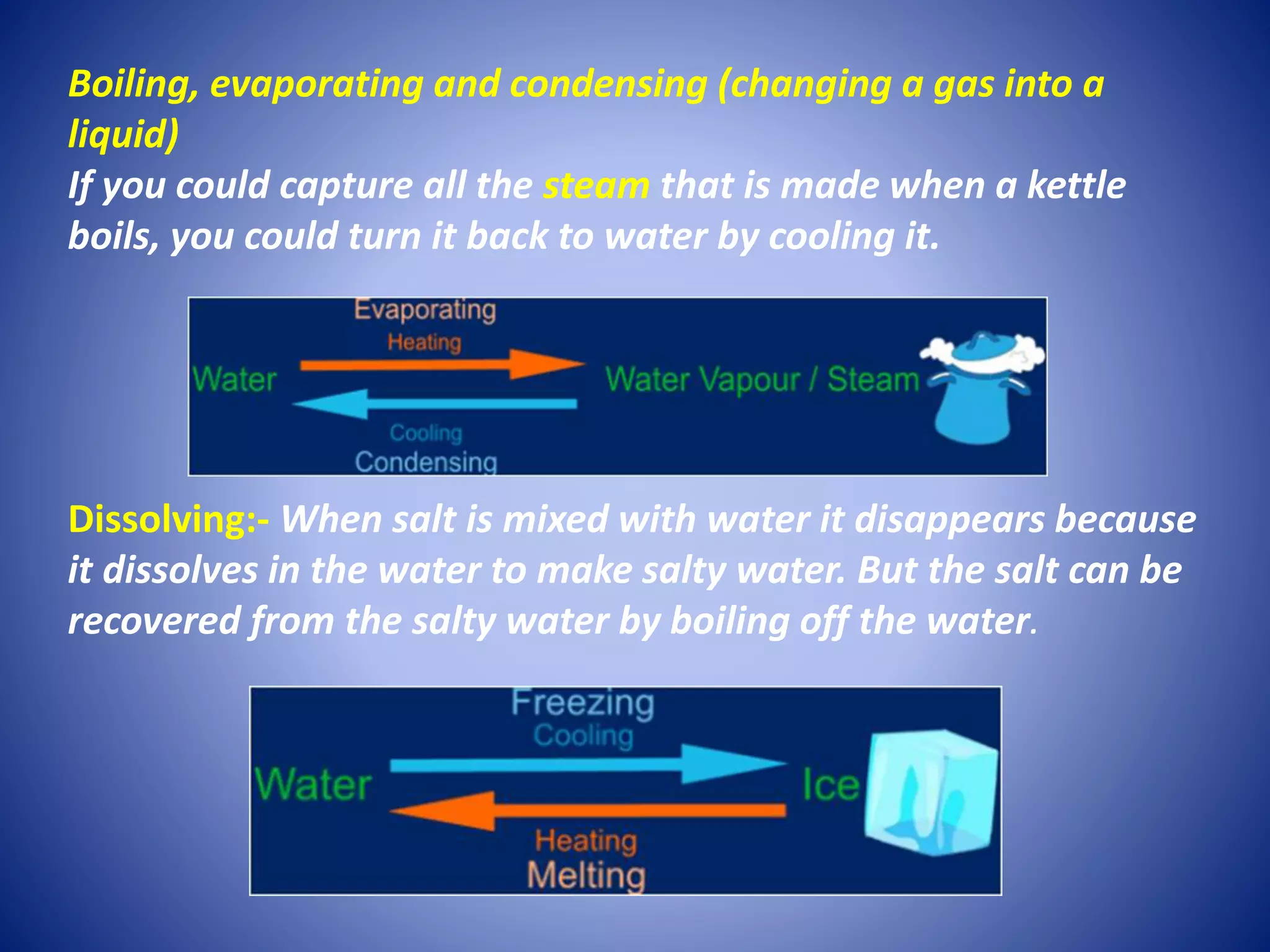
This document discusses physical and chemical changes. It defines irreversible changes as permanent changes that cannot be undone, involving chemical reactions that produce new materials. Reversible changes are changes that can be undone, involving only physical changes in state or form. Some examples of each are provided, like baking being irreversible while melting and freezing are reversible. Criteria for identifying chemical versus physical changes are outlined. The document concludes by introducing an activity to set up crystal geodes.