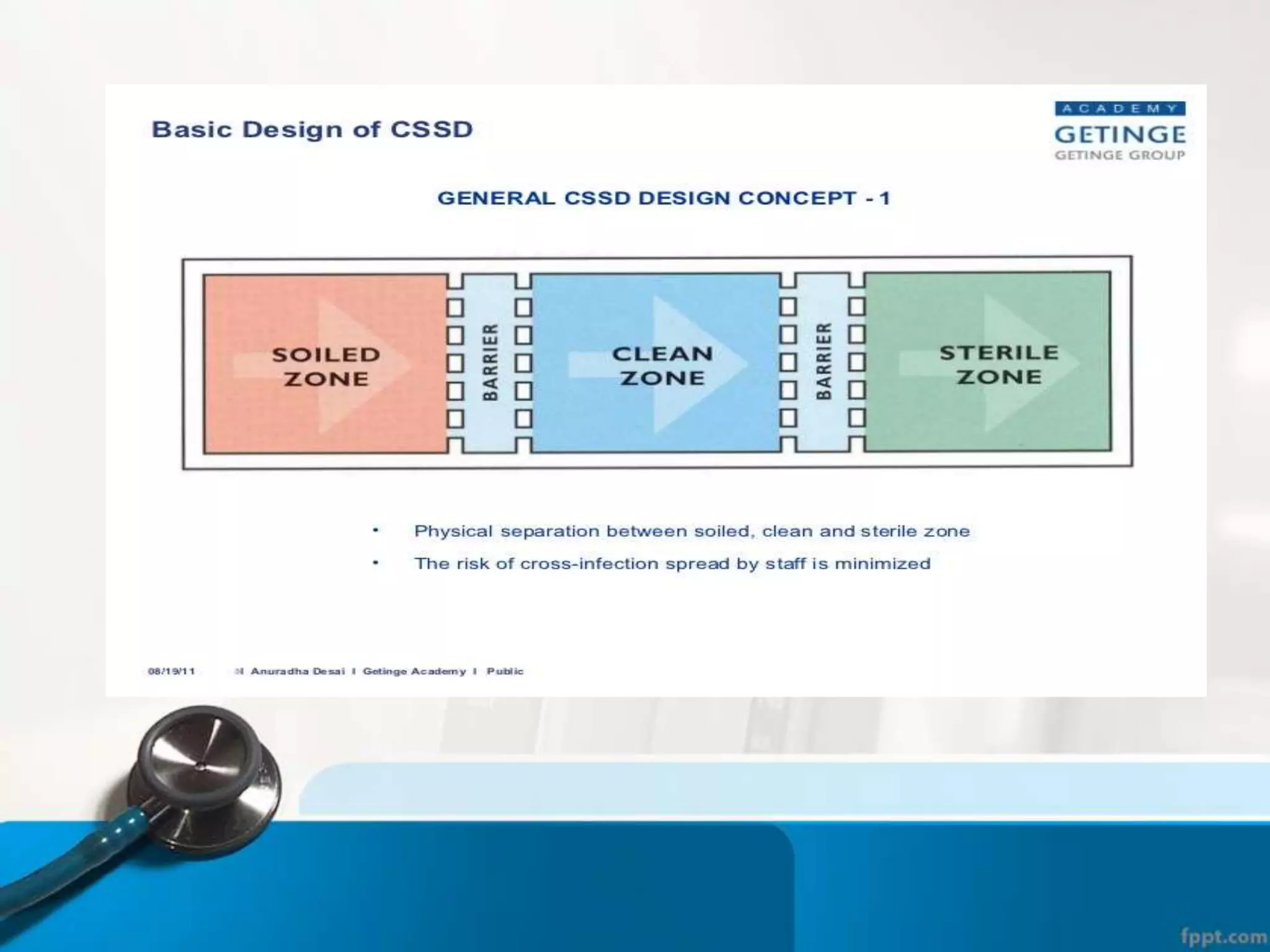
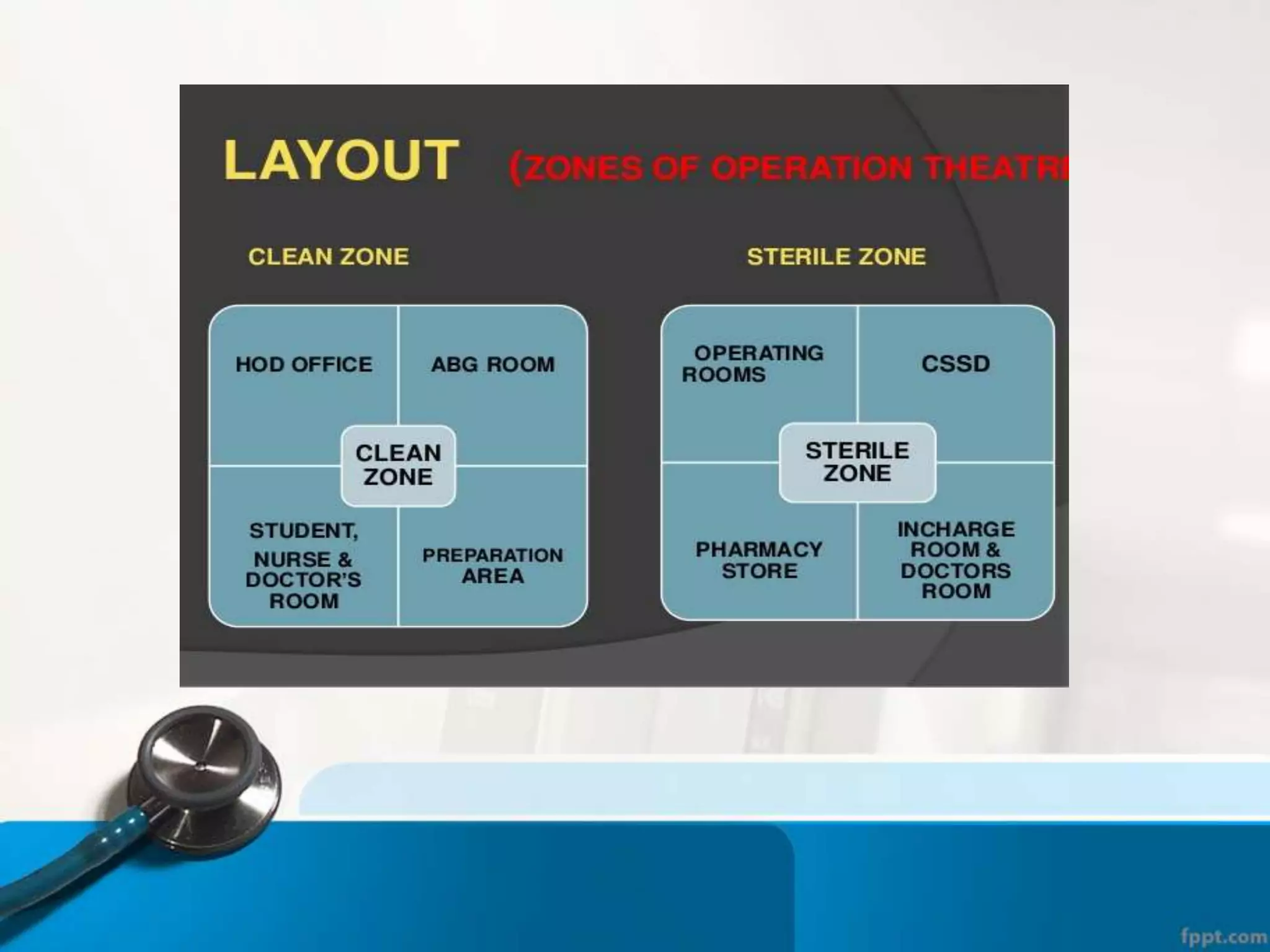
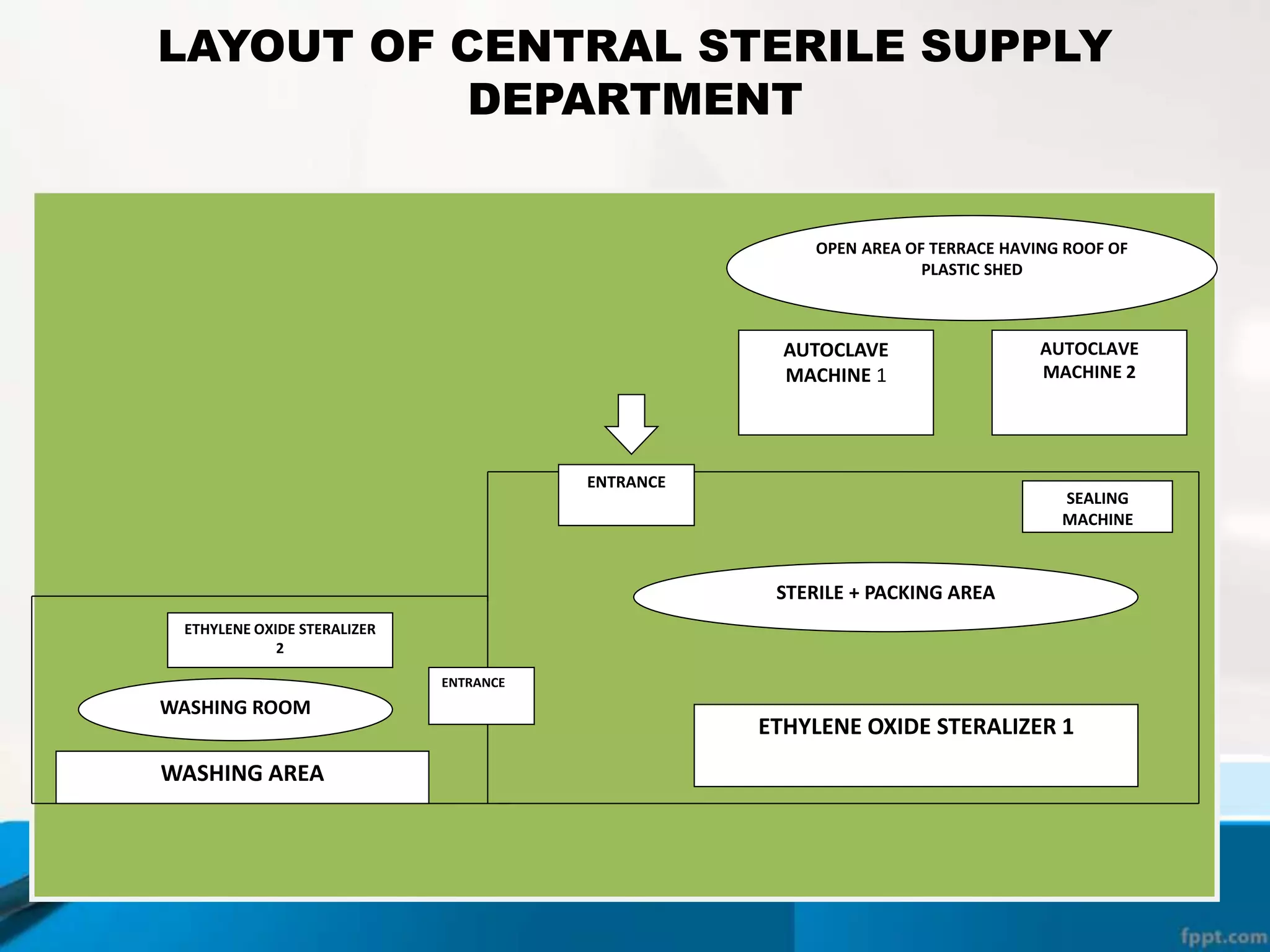
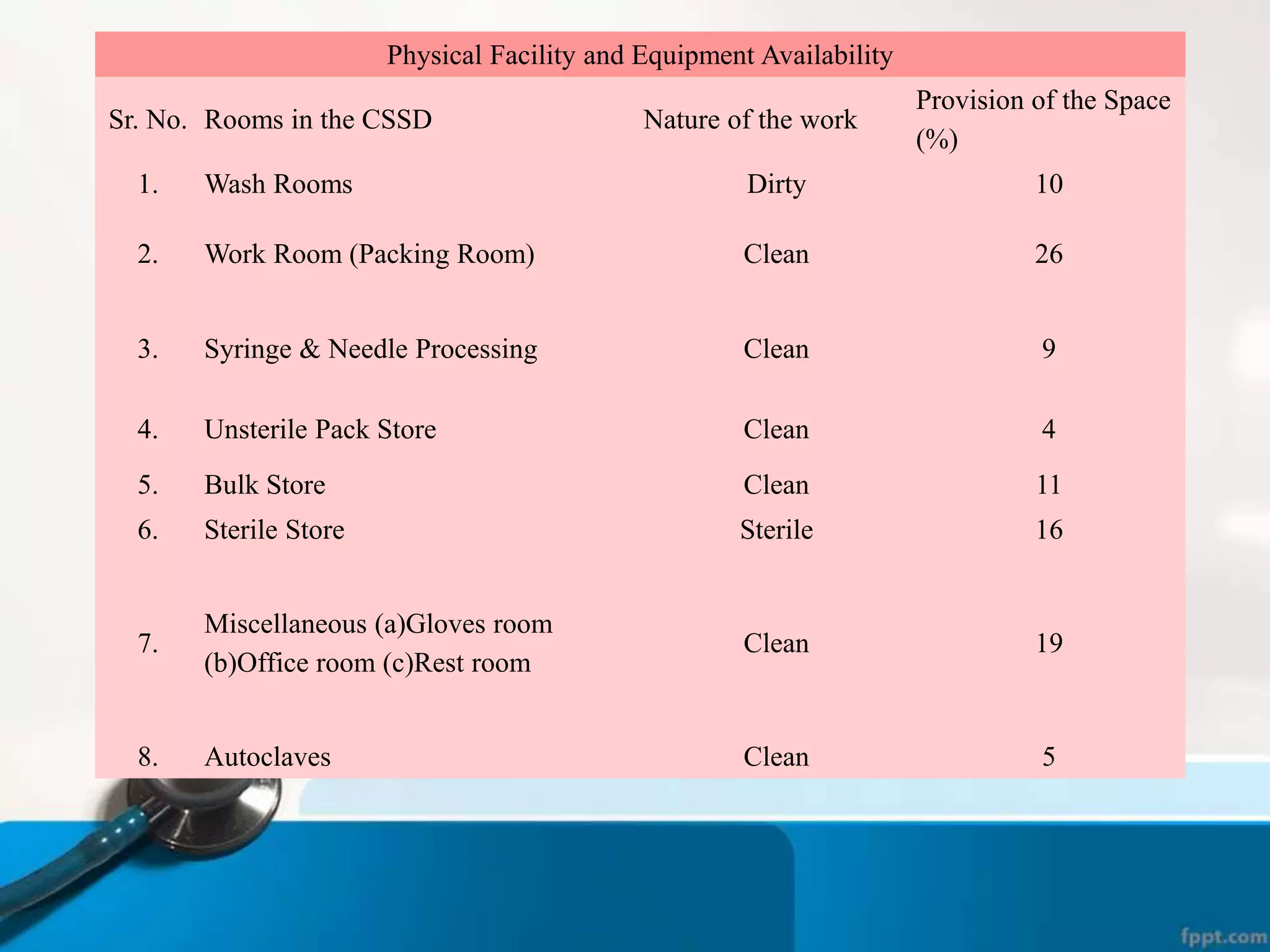
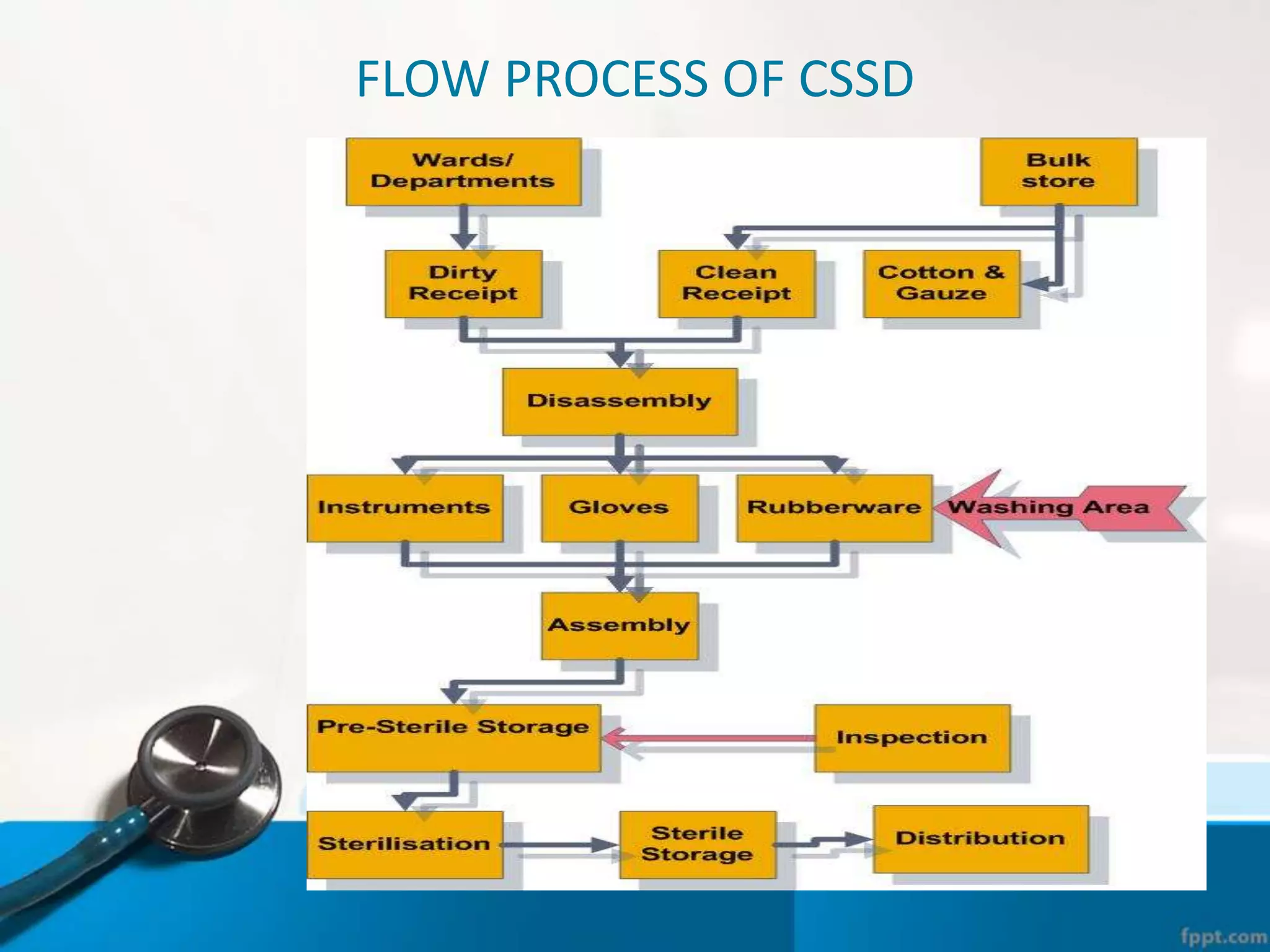
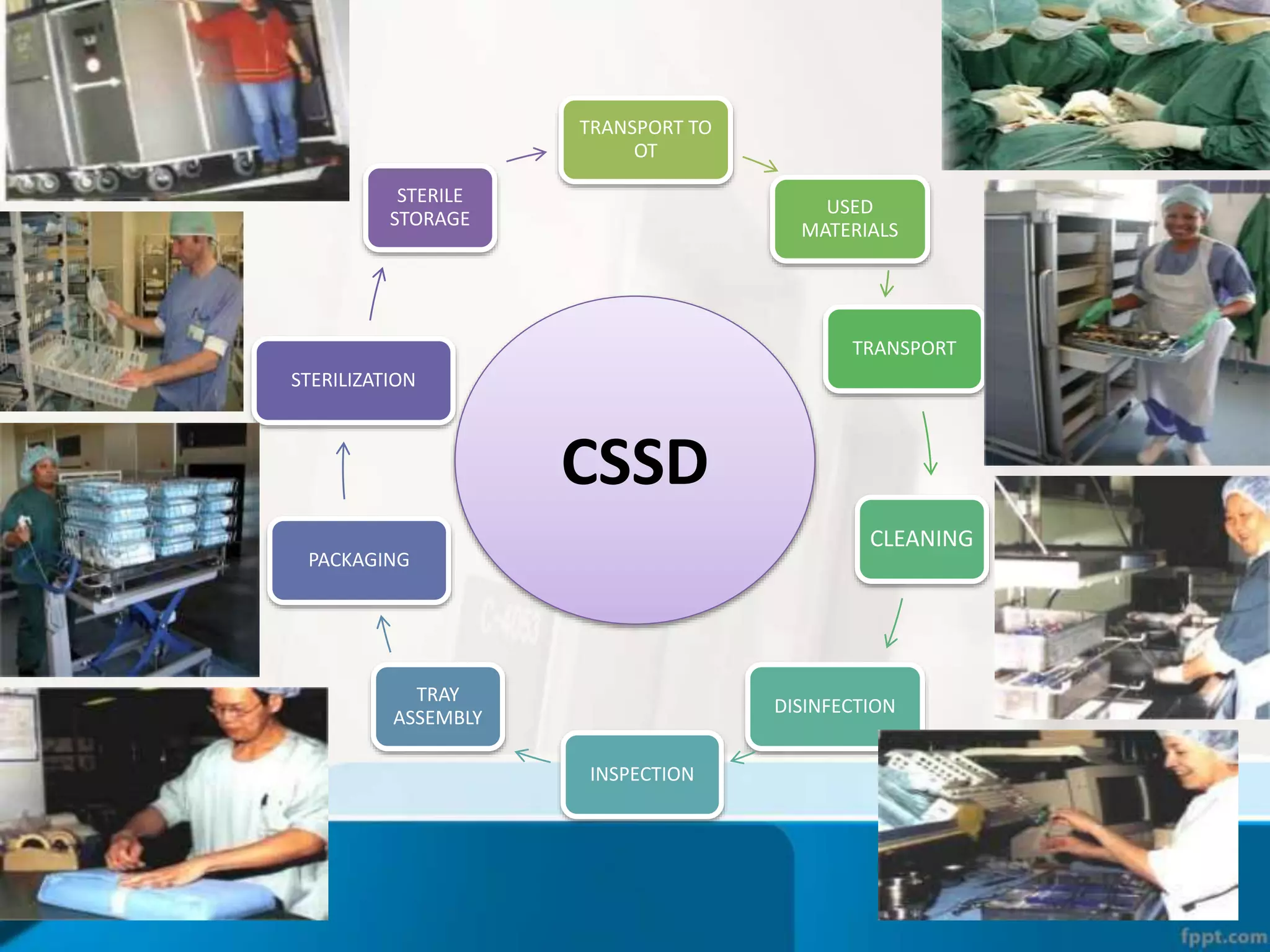
The document describes the Central Sterile Supply Department (CSSD) in a hospital. CSSD receives, cleans, sterilizes, and distributes medical supplies and equipment to different departments. It aims to provide sterilized materials and reduce hospital infections. CSSD has four zones - unclean/washing, assembly/packing, sterilization, and storage. Materials flow through receiving, cleaning, packing, sterilizing, storing and distribution. CSSD is staffed by a manager, technicians, assistants and clerks and uses equipment like autoclaves and ethylene oxide sterilizers.