Key Concepts Unit A Chemistry 30
•Download as PPTX, PDF•
1 like•1,059 views
Report
Share
Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Chapter 21.1 : Functional Groups and Classes of Organic Compounds

Chapter 21.1 : Functional Groups and Classes of Organic Compounds
Similar to Key Concepts Unit A Chemistry 30
Similar to Key Concepts Unit A Chemistry 30 (20)
Chapter-Organic-Chemistry-class-10-important-Questions.pdf

Chapter-Organic-Chemistry-class-10-important-Questions.pdf
Introductiontoorganicchemistry 090518040648 Phpapp02

Introductiontoorganicchemistry 090518040648 Phpapp02
Recently uploaded
Recently uploaded (20)
ASRock Industrial FDO Solutions in Action for Industrial Edge AI _ Kenny at A...

ASRock Industrial FDO Solutions in Action for Industrial Edge AI _ Kenny at A...
State of the Smart Building Startup Landscape 2024!

State of the Smart Building Startup Landscape 2024!
Long journey of Ruby Standard library at RubyKaigi 2024

Long journey of Ruby Standard library at RubyKaigi 2024
Intro in Product Management - Коротко про професію продакт менеджера

Intro in Product Management - Коротко про професію продакт менеджера
Your enemies use GenAI too - staying ahead of fraud with Neo4j

Your enemies use GenAI too - staying ahead of fraud with Neo4j
Powerful Start- the Key to Project Success, Barbara Laskowska

Powerful Start- the Key to Project Success, Barbara Laskowska
TEST BANK For, Information Technology Project Management 9th Edition Kathy Sc...

TEST BANK For, Information Technology Project Management 9th Edition Kathy Sc...
Using IESVE for Room Loads Analysis - UK & Ireland

Using IESVE for Room Loads Analysis - UK & Ireland
Linux Foundation Edge _ Overview of FDO Software Components _ Randy at Intel.pdf

Linux Foundation Edge _ Overview of FDO Software Components _ Randy at Intel.pdf
The Value of Certifying Products for FDO _ Paul at FIDO Alliance.pdf

The Value of Certifying Products for FDO _ Paul at FIDO Alliance.pdf
Integrating Telephony Systems with Salesforce: Insights and Considerations, B...

Integrating Telephony Systems with Salesforce: Insights and Considerations, B...
FDO for Camera, Sensor and Networking Device – Commercial Solutions from VinC...

FDO for Camera, Sensor and Networking Device – Commercial Solutions from VinC...
A Business-Centric Approach to Design System Strategy

A Business-Centric Approach to Design System Strategy
Key Concepts Unit A Chemistry 30
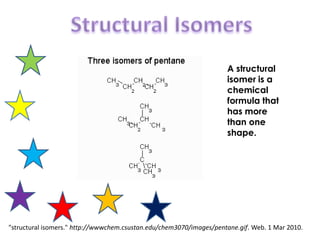
- 1. Structural Isomers A structural isomer is a chemical formula that has more than one shape. "structural isomers." http://wwwchem.csustan.edu/chem3070/images/pentane.gif. Web. 1 Mar 2010.
- 2. Saturated/Unsaturated Hydrocarbons Saturated hydrocarbons have single bonds and cannot bond with compounds anymore. Unsaturated hydrocarbons have double or triple bonds and can bond with compounds.
- 3. Halogenated Hydrocarbons Are produced When a Hydrocarbon has one or more hydrogen group replaced with a Halogen(group 17) Can help create a saturated hydrocarbon by breaking double or triple bonds. http://www.youtube.com/watch?v=1qGPWdm2MlI (watch to 1:50)
- 4. Carboxylic Acids The Basic Structure is R-COOH (R can be replaced by a hydrogen) They are used in an esterfication reaction with an alcohol When naming you change the ending to “oic” acid (methane would be methanoic acid) They have hydrogen bonds increasing their boiling points.
- 5. Structuralformulas The structural formula of a chemical compound is a graphical representation showing how the atoms are arranged. This is a structural formula where atoms are arranged forming a compound Structural formulas rock my world!!! http://en.wikipedia.org/wiki/Structural_formula http://www.1-formula.com/upl/Image/800px-Acetone-structural.png
- 6. Esterification http://en.wikipedia.org/wiki/Esterification This is an Esterification reaction I love Esterification reactions because they smell good Esterification is the general name for a chemical reaction in which two reactants (typically an alcohol and a carboxylic acid) form an ester as the reaction product.
- 7. Addition Reaction An addition reaction is the reaction between two or more unsaturated hydrocarbons to produce one large molecule.
- 8. Substitution Reaction When a carbon-hydrogen bond breaking occurs in an alkane or aromatic organic reaction An example is the reaction in which the chlorine atom in chloromethane is displaced by the hydroxide ion to form methanol CH3Cl + -OH CH3OH + Cl-
- 9. Addition and substitution reaction Video http://www.youtube.com/watch?v=nVDUB4mWJ8M
- 11. A Complete Combustion reaction is when the reactant will burn in oxygen producing a limited number of products.
- 12. An incomplete combustion reaction is when there isn’t enough oxygen to react with.C?H? + O2 CO2 + H2O C?H? + O2 CO + H2O "combustion reactions.". http://upload.wikimedia.org/wikipedia/commons/3/3c/Et_baal.jpg. Web. 1 Mar 2010
- 13. Monomers monomer - a simple compound whose molecules can join together to form polymers.
- 14. Polymers Polymers are created when two or more monomers are linked together in a chain like pattern
- 15. Polymerization Polymerization - The chemical process, normally needs the aid of a catalyst, to form a polymer by bonding together monomers. http://www.youtube.com/watch?v=LJ5hjUeZt7U&feature=related
- 16. Polymers are made from Monomers http://www.youtube.com/watch?v=Jpv2RUjNFjo