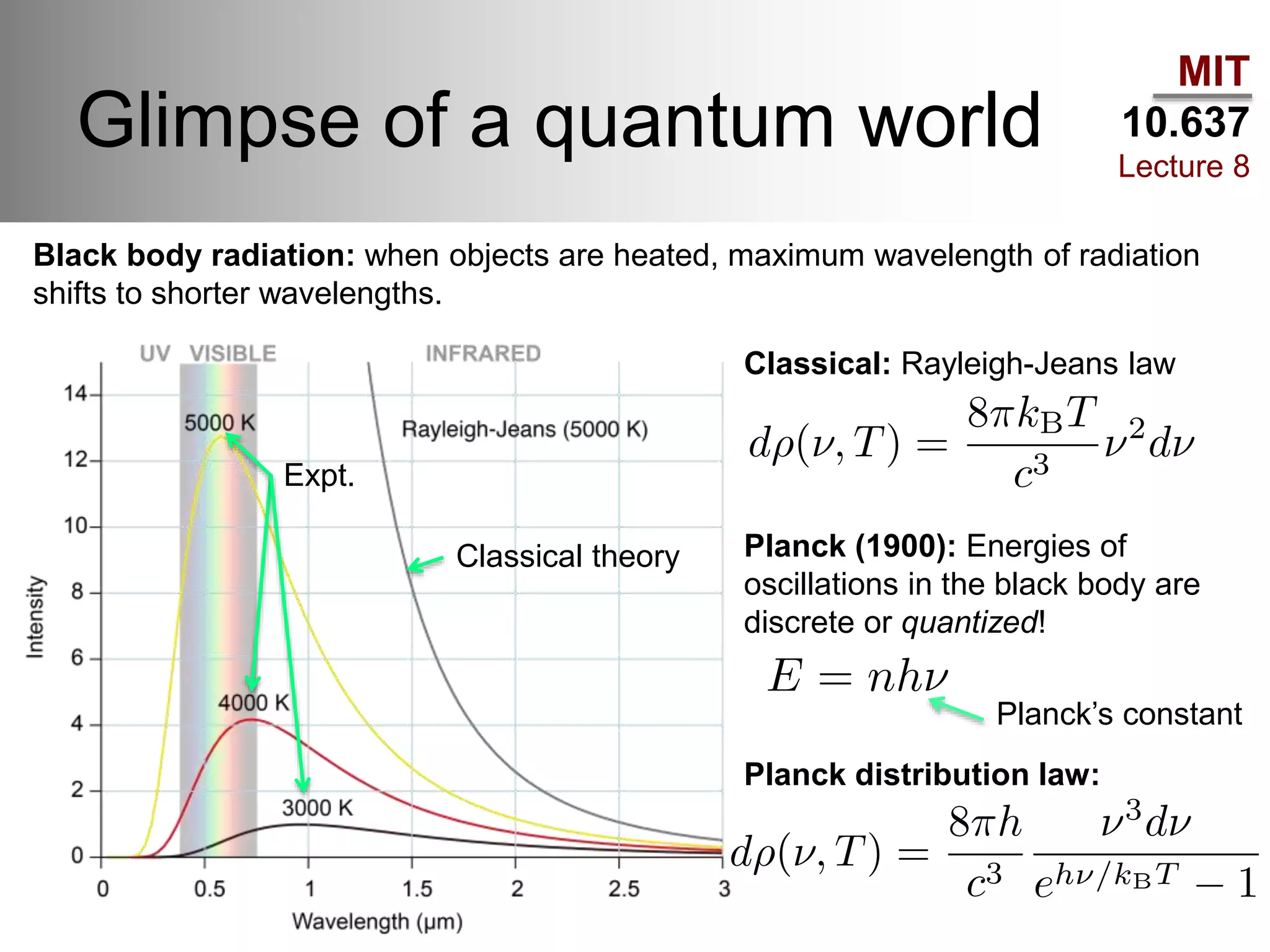
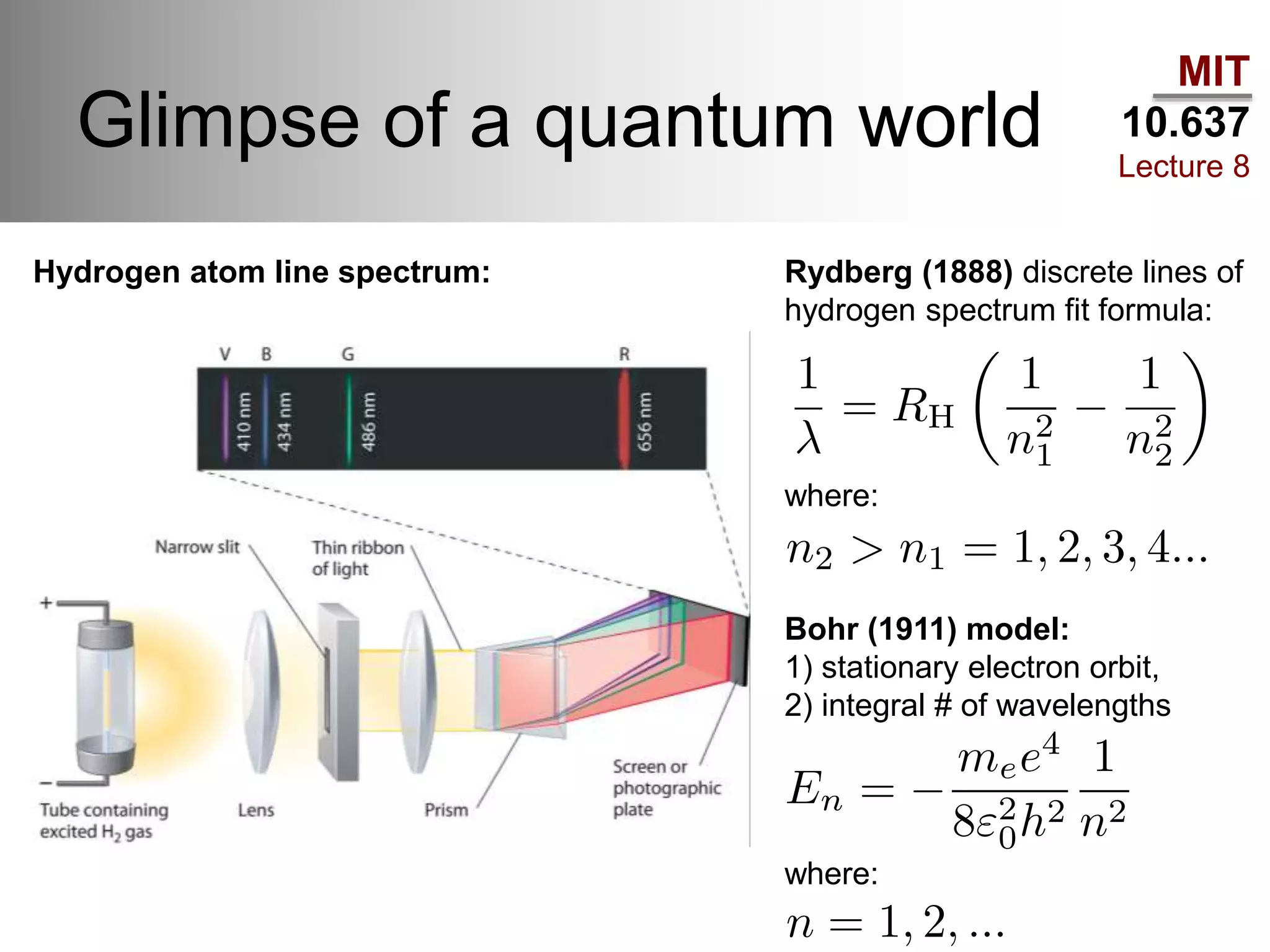
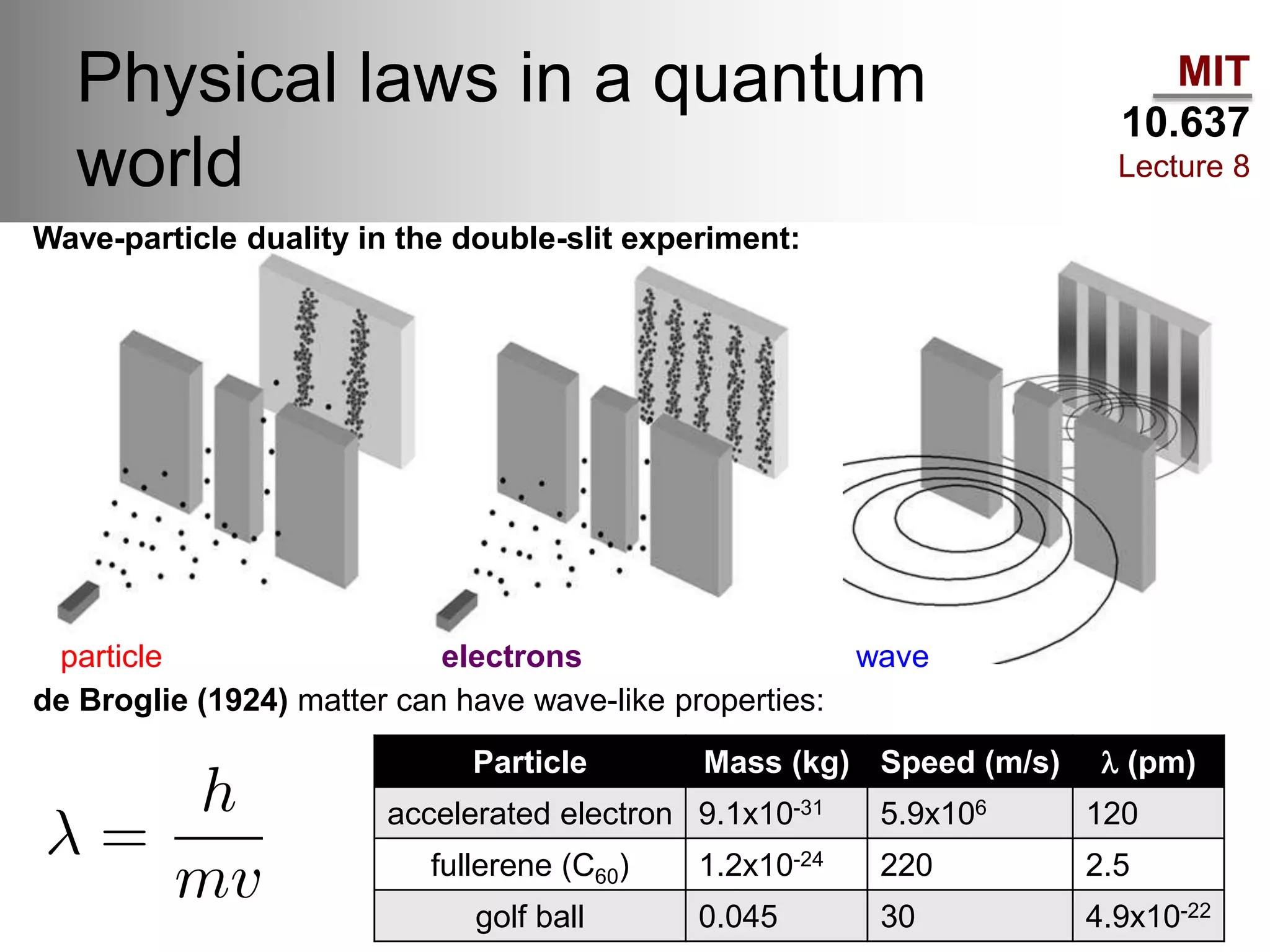
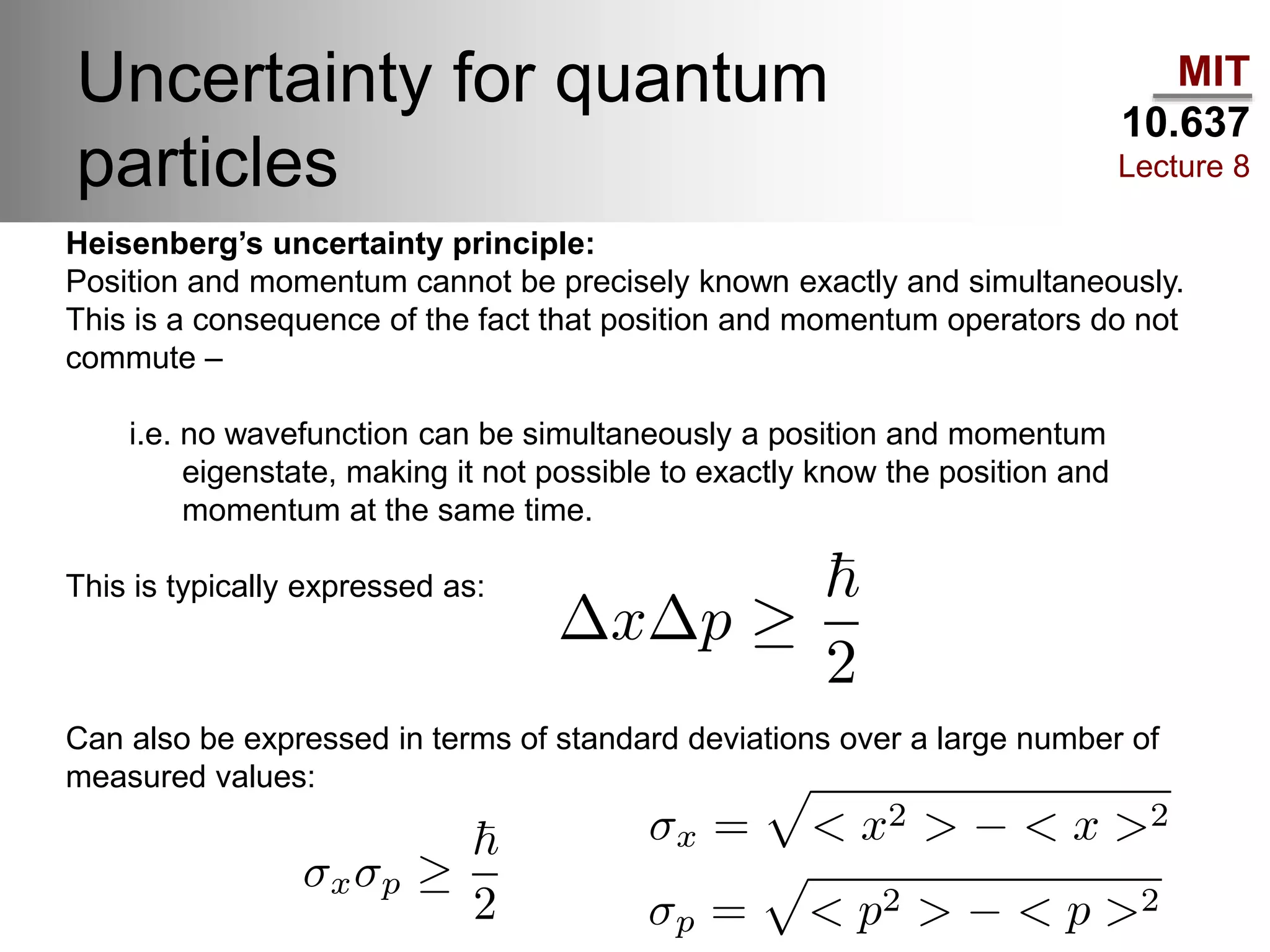
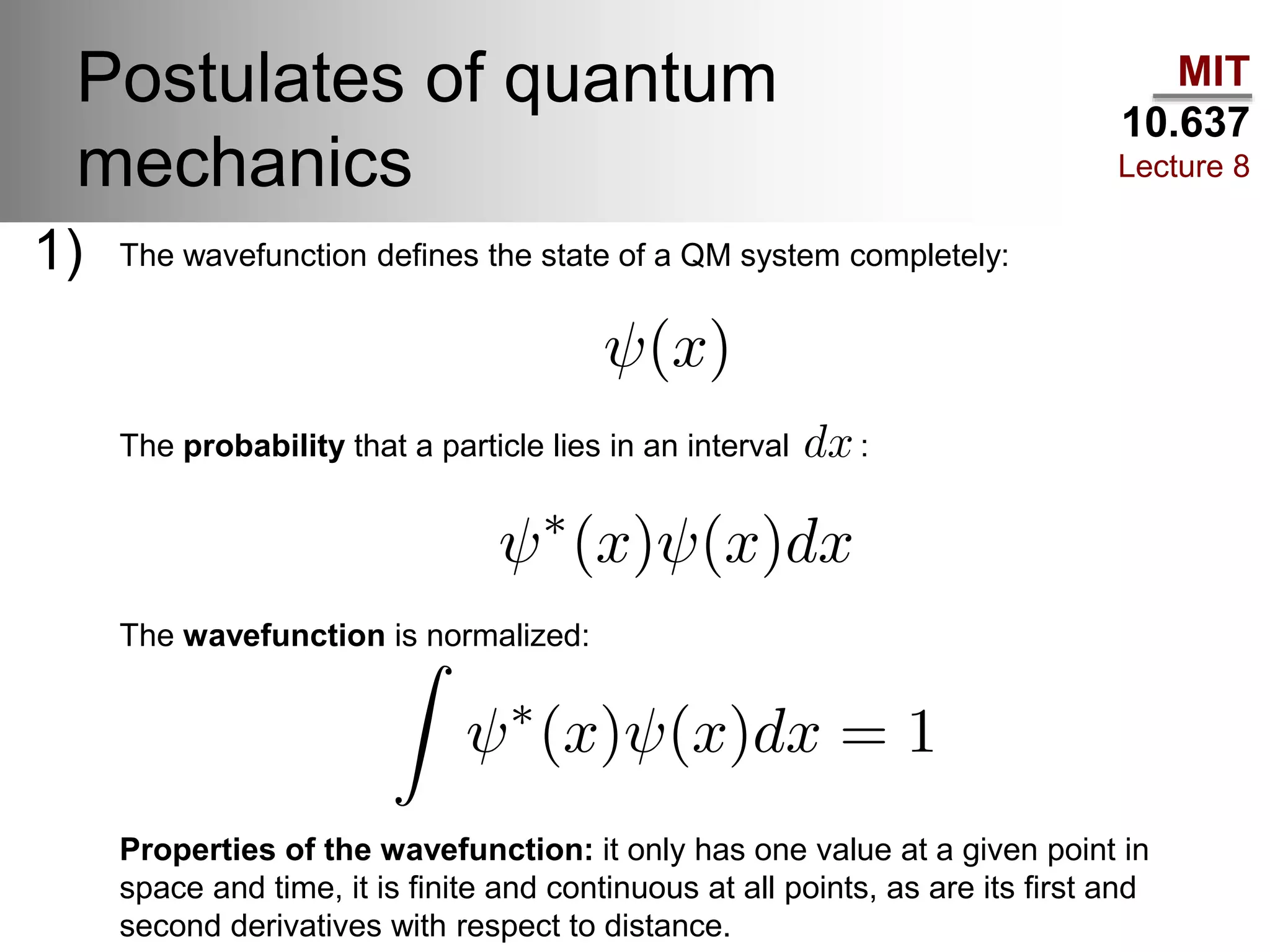
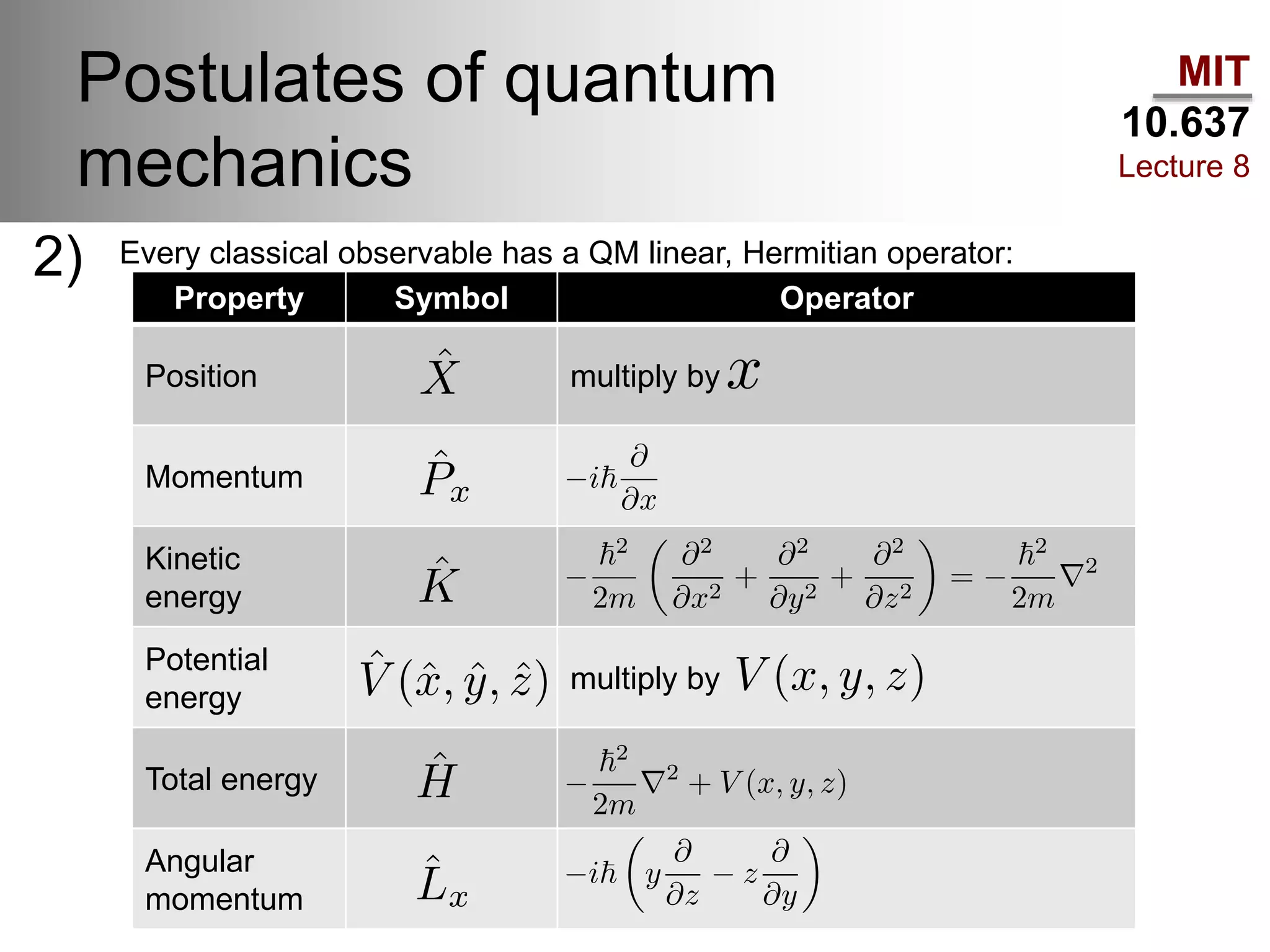
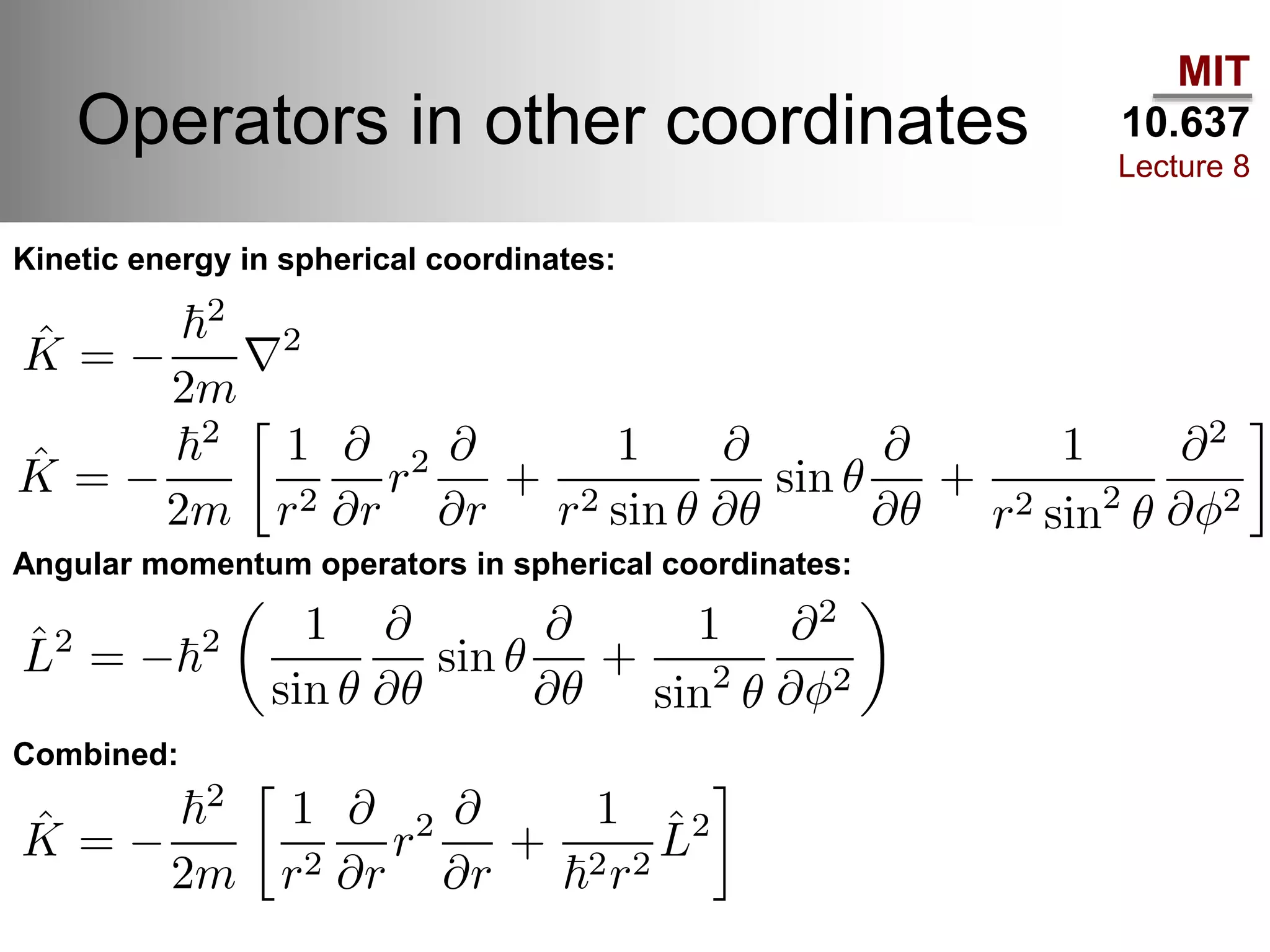
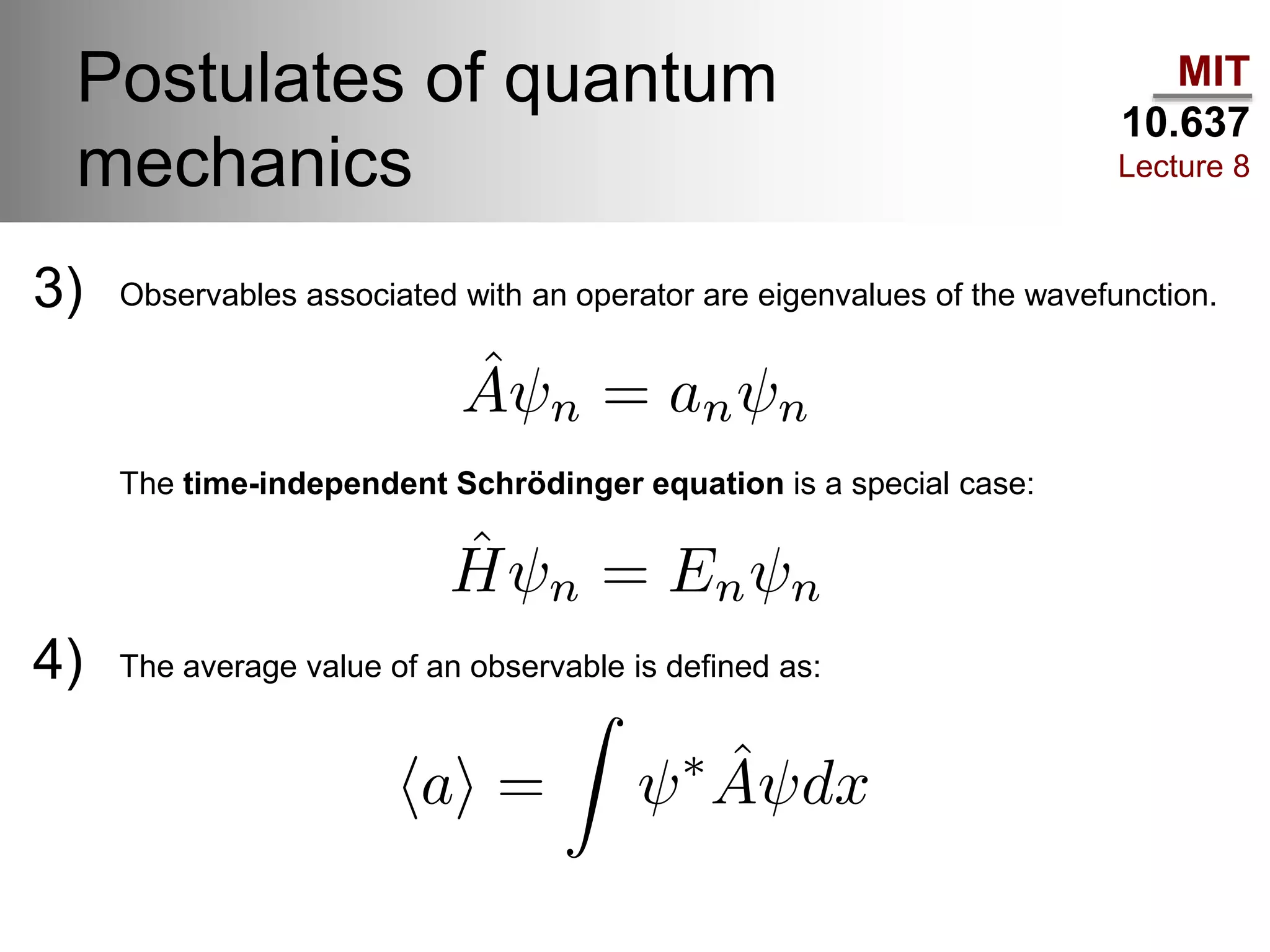
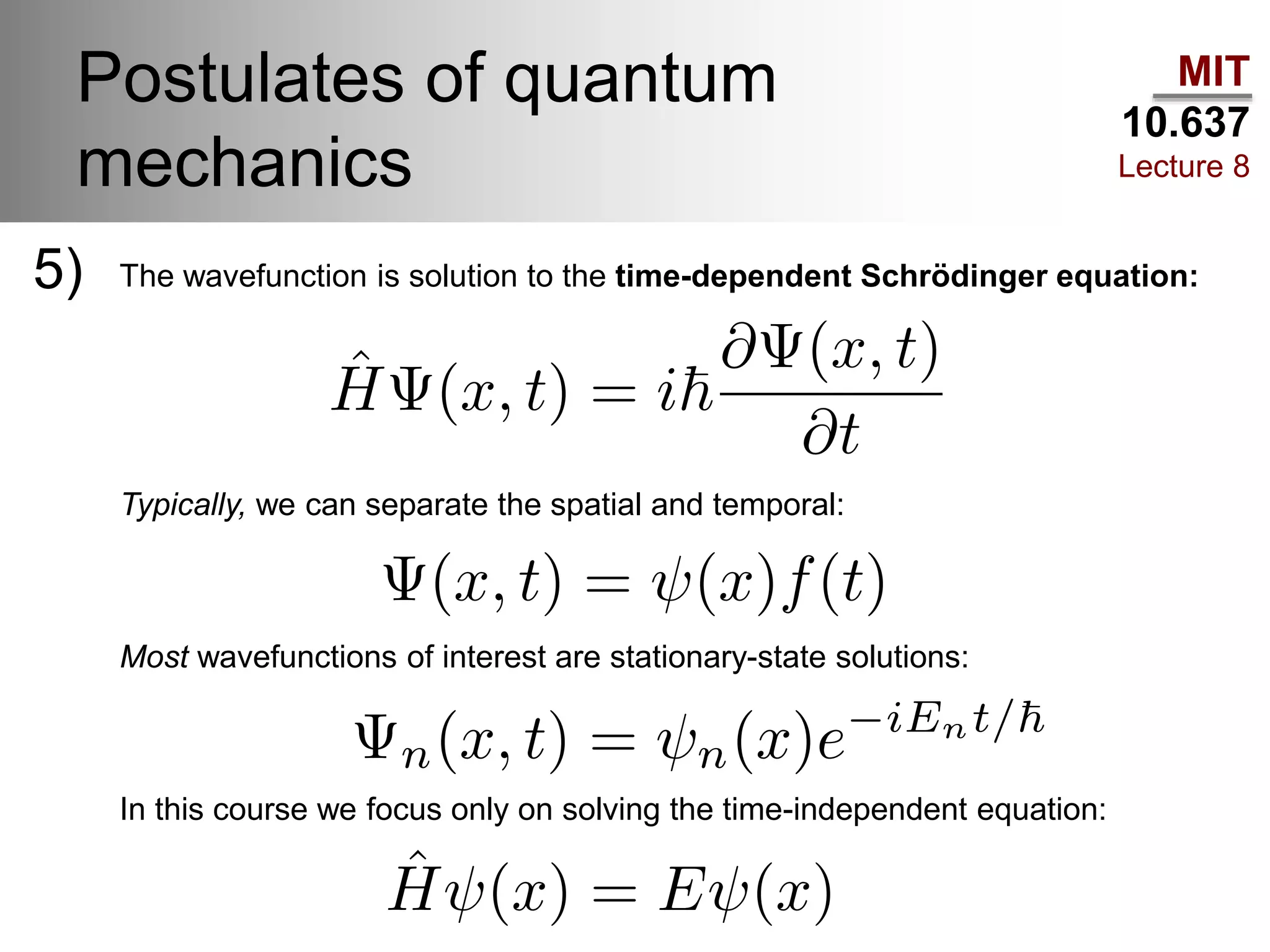
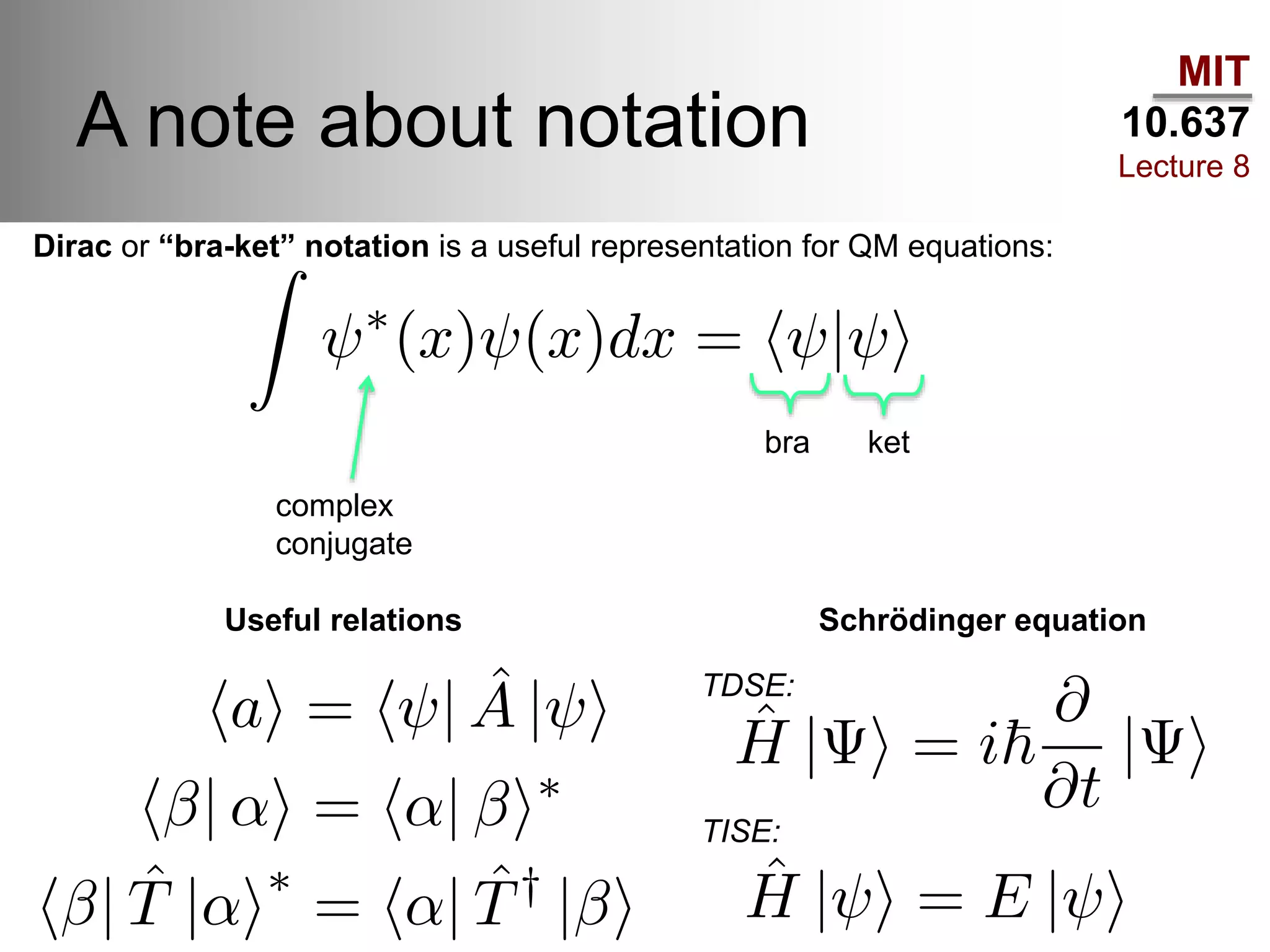
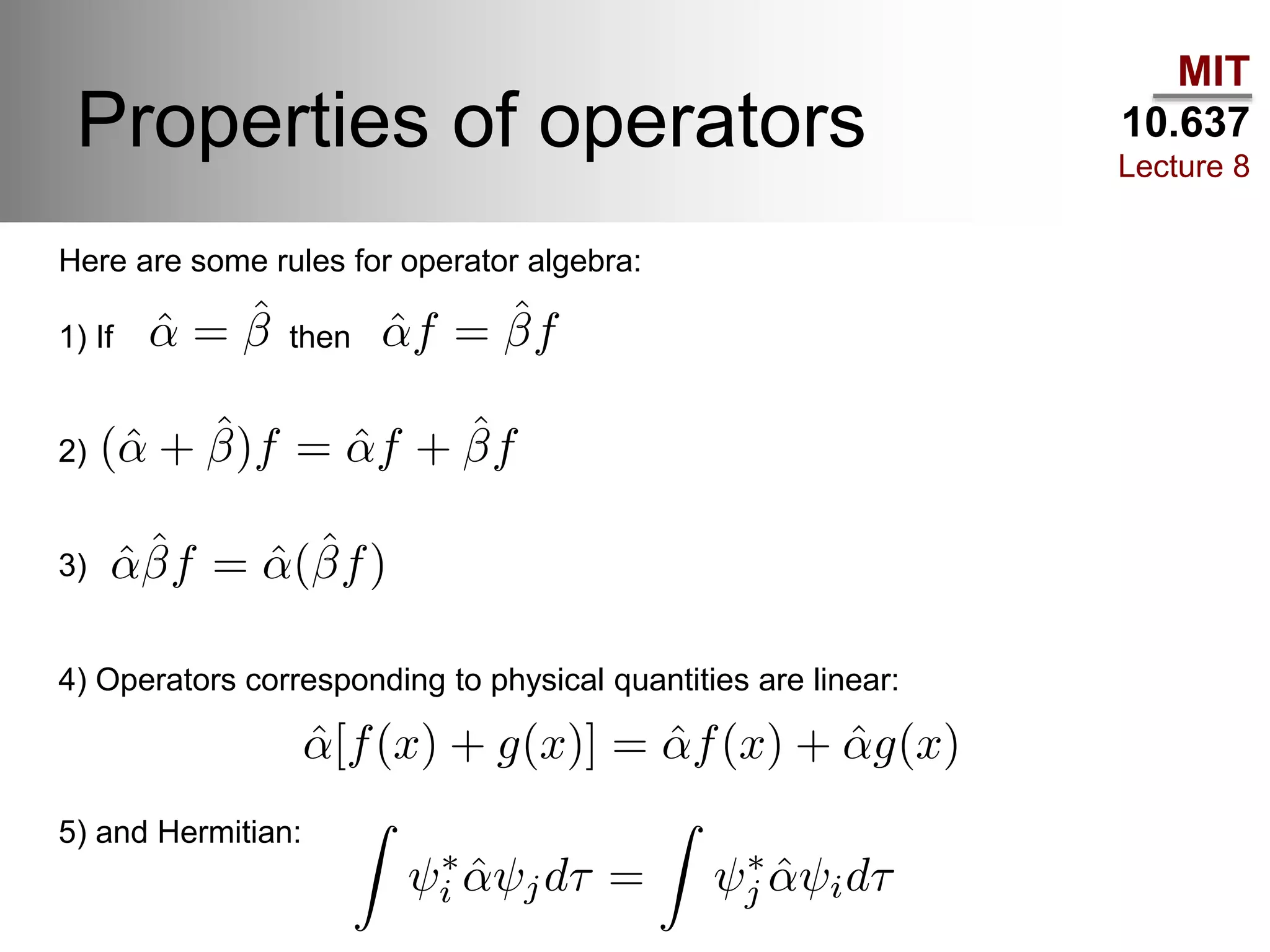
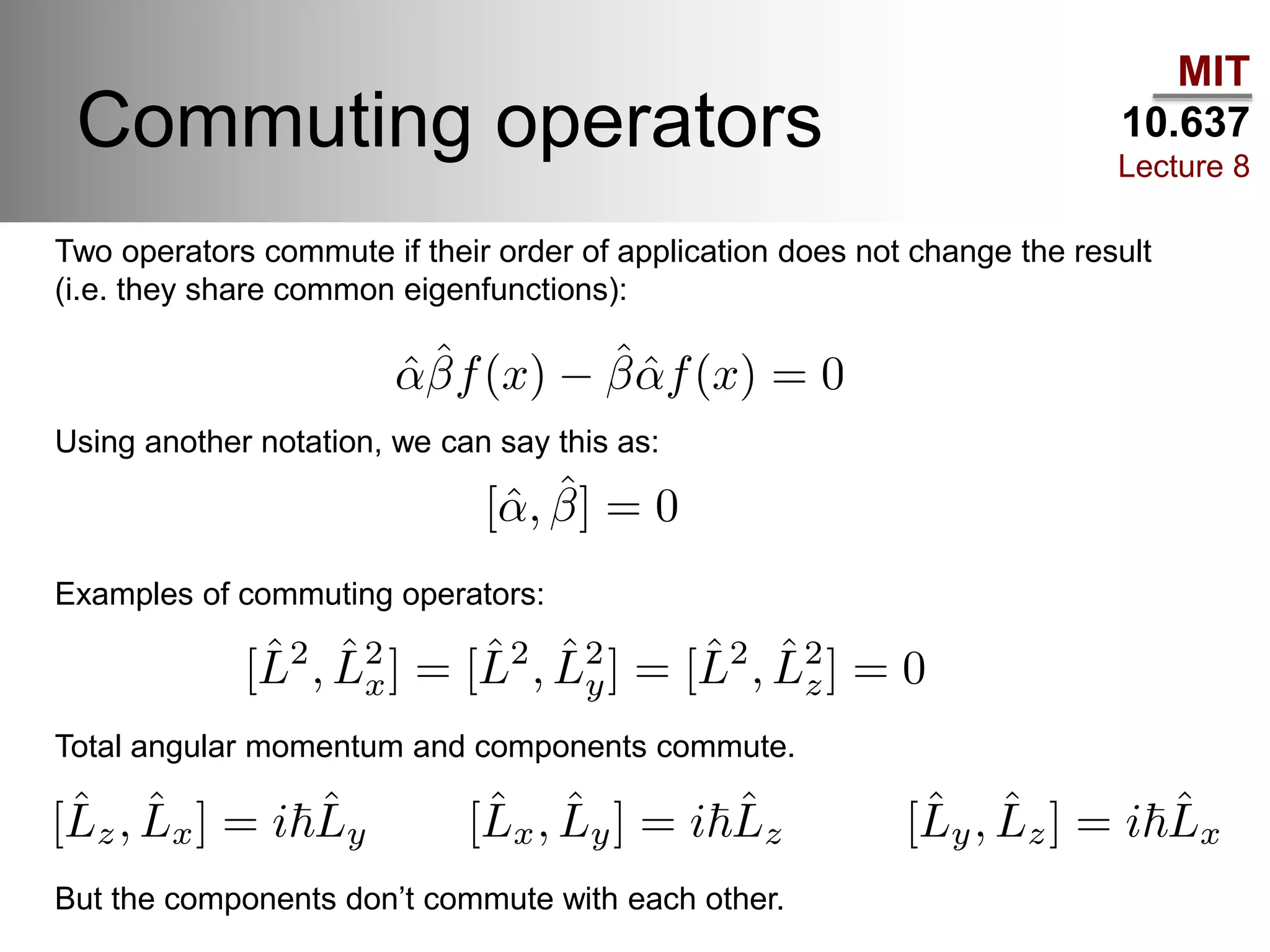
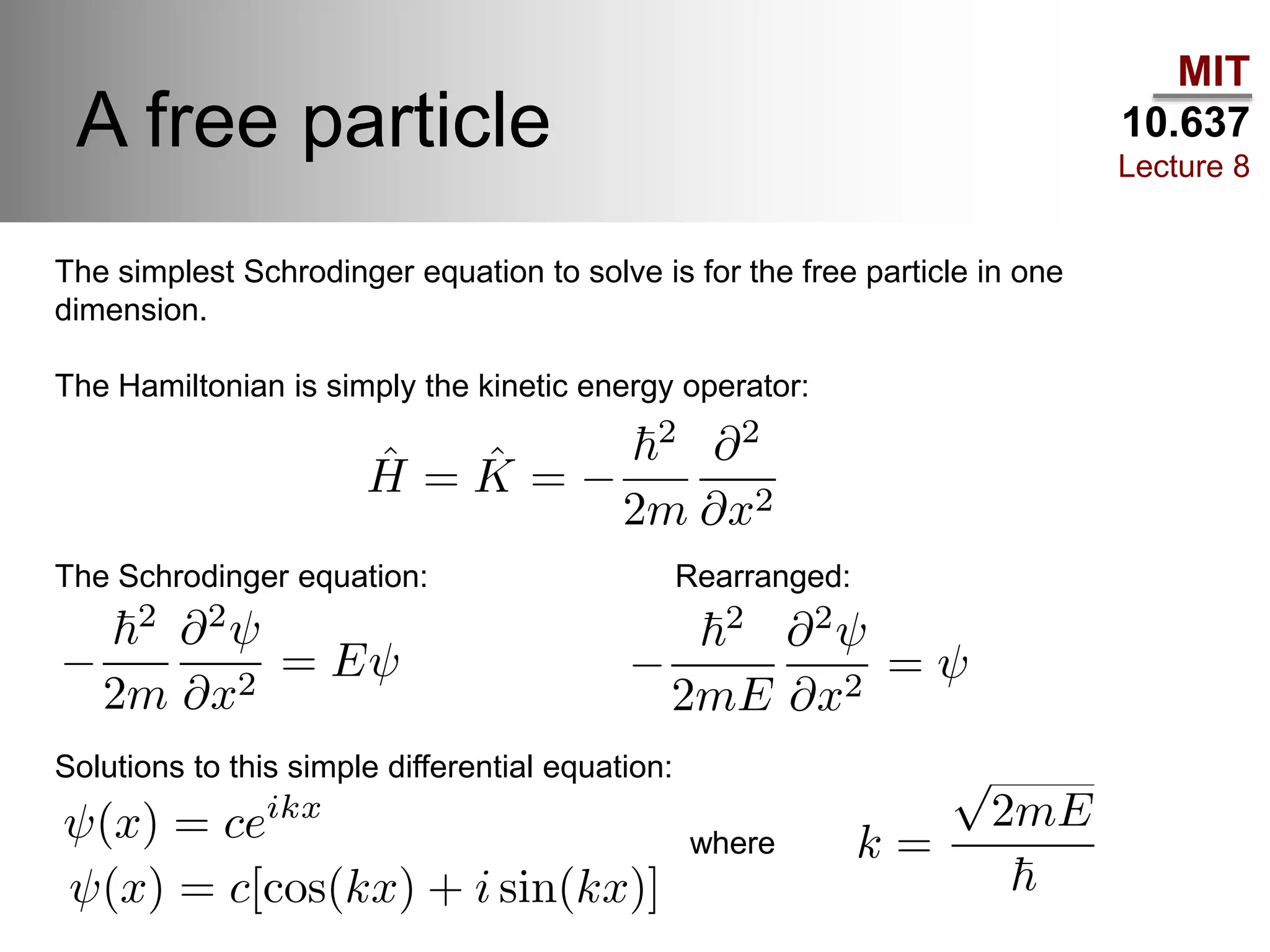
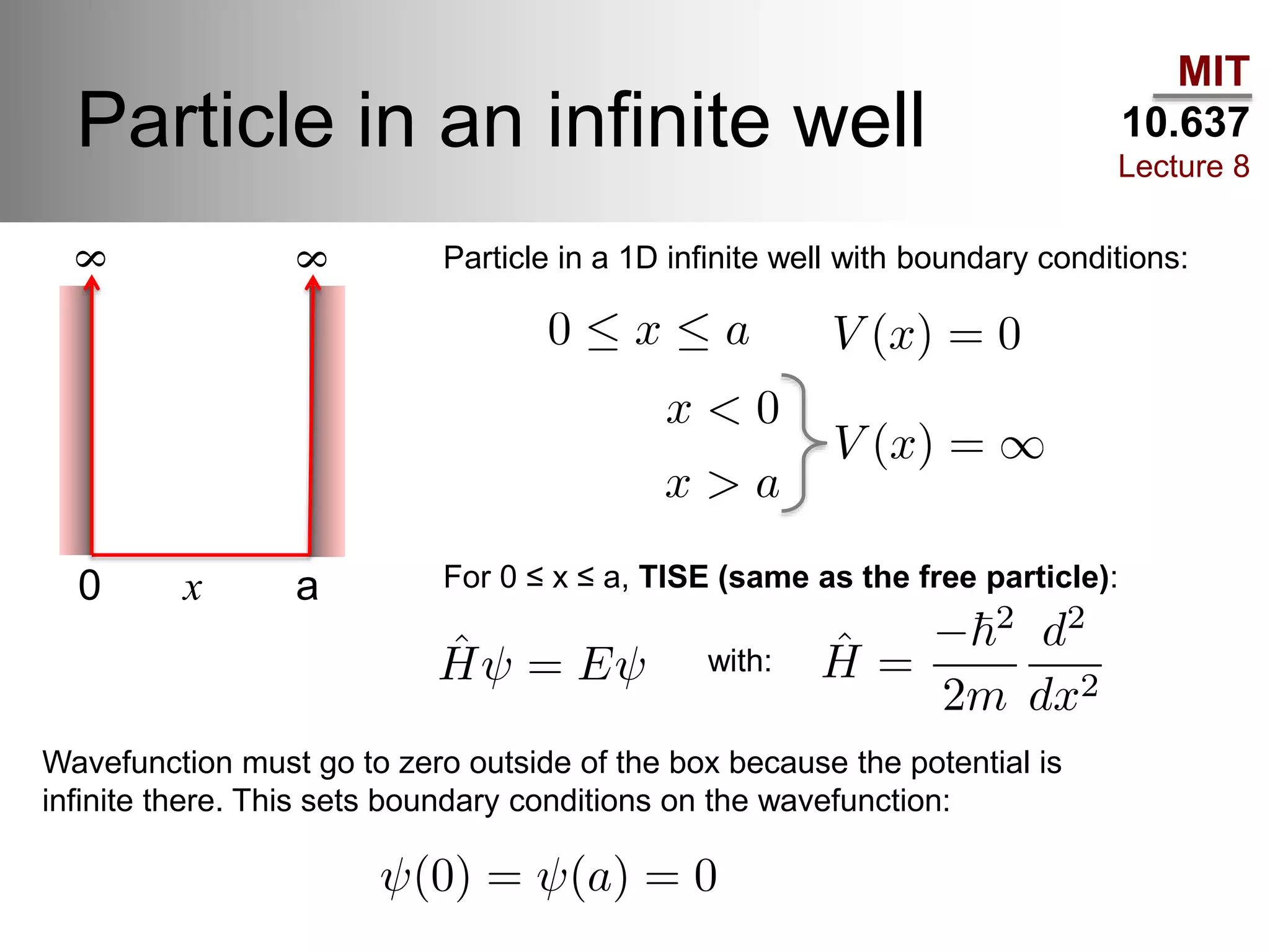
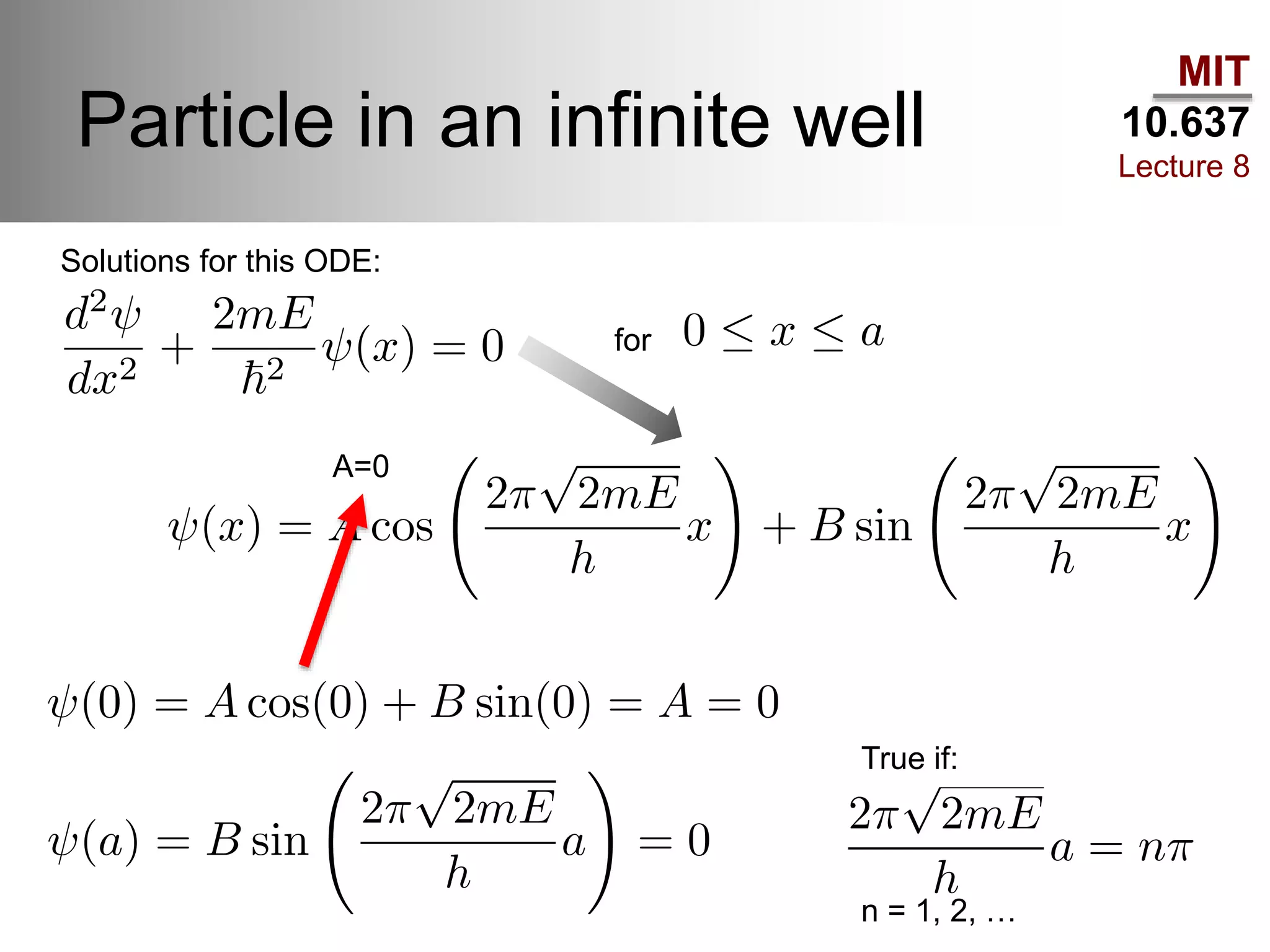
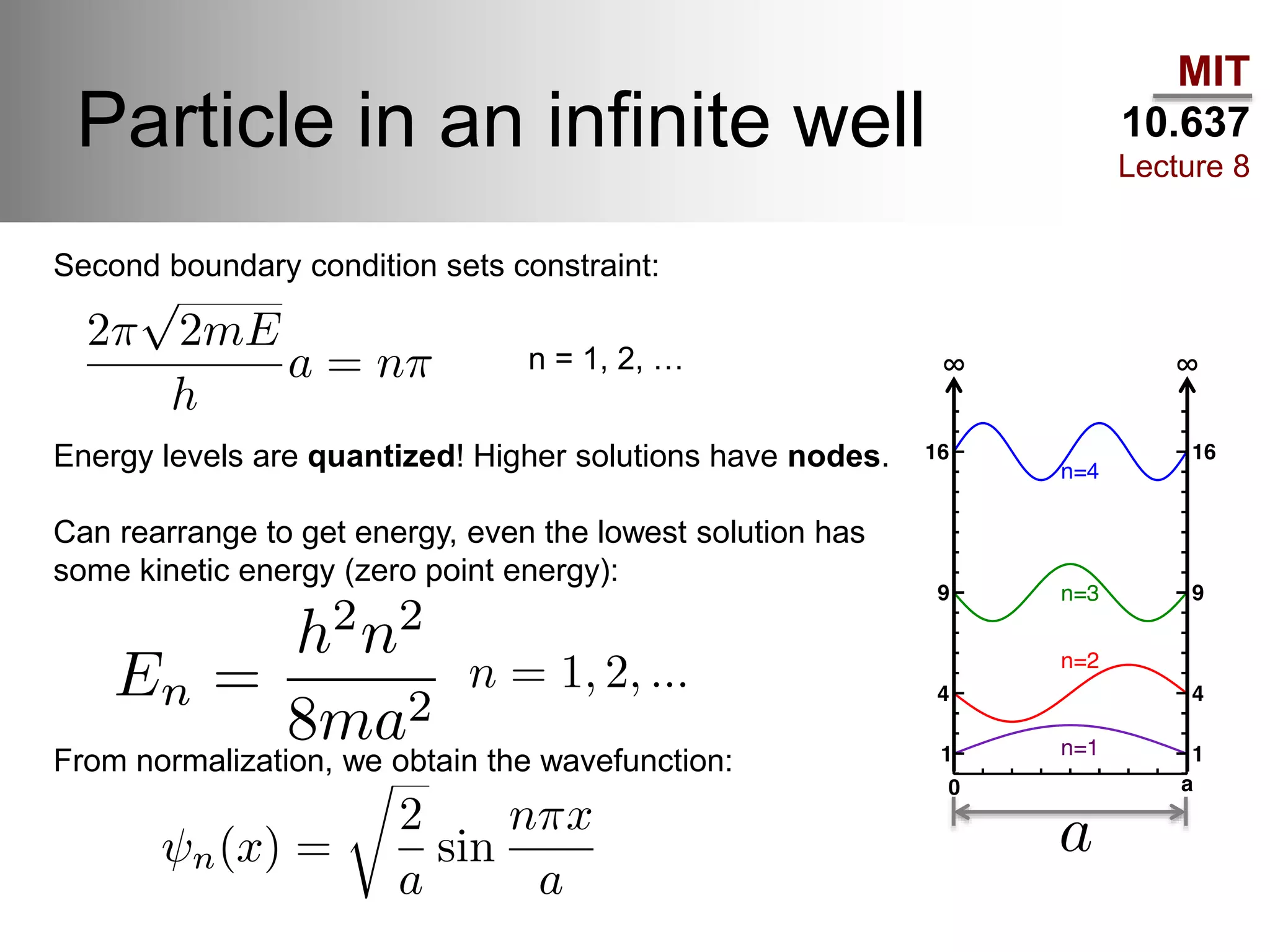
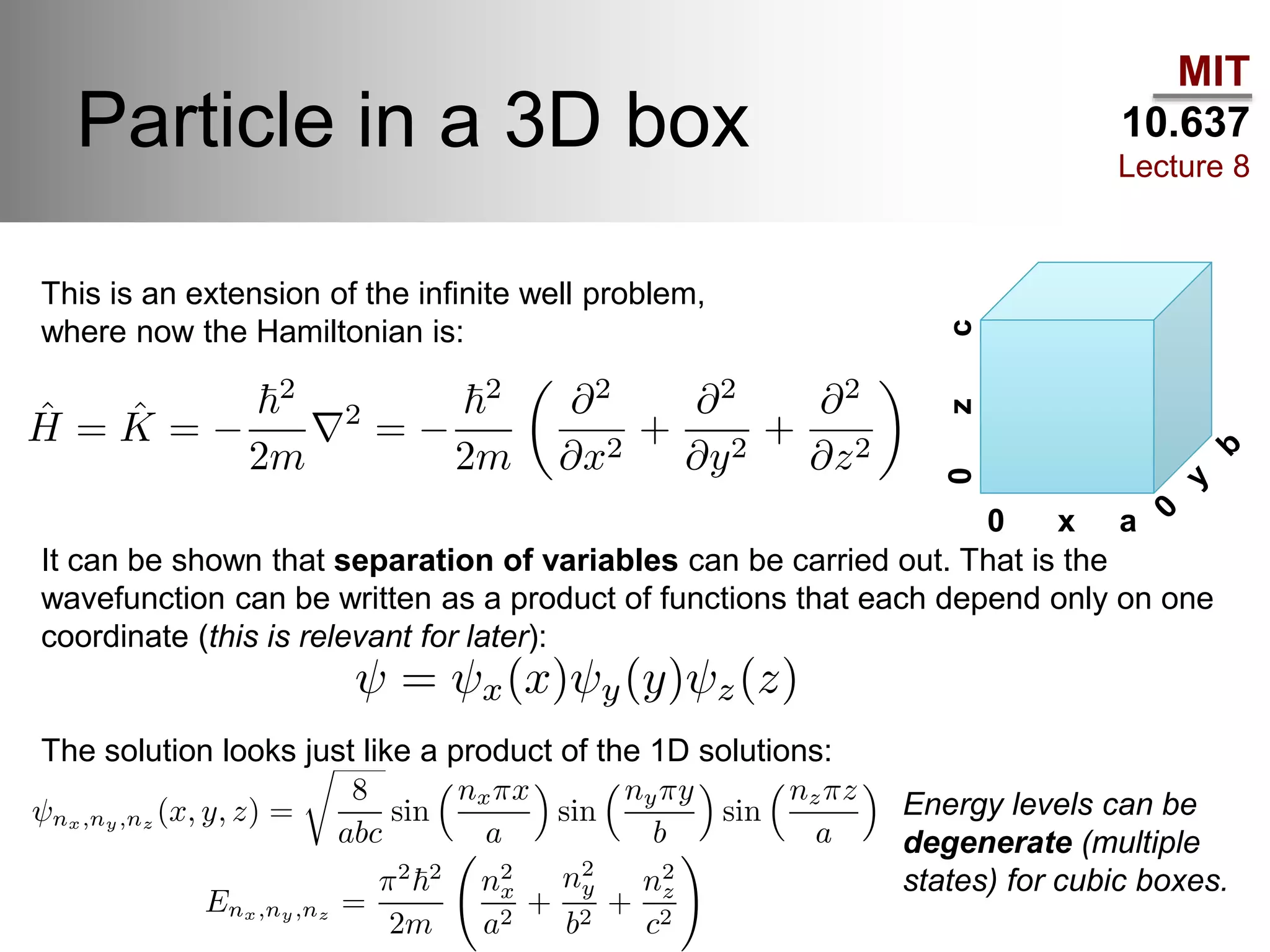
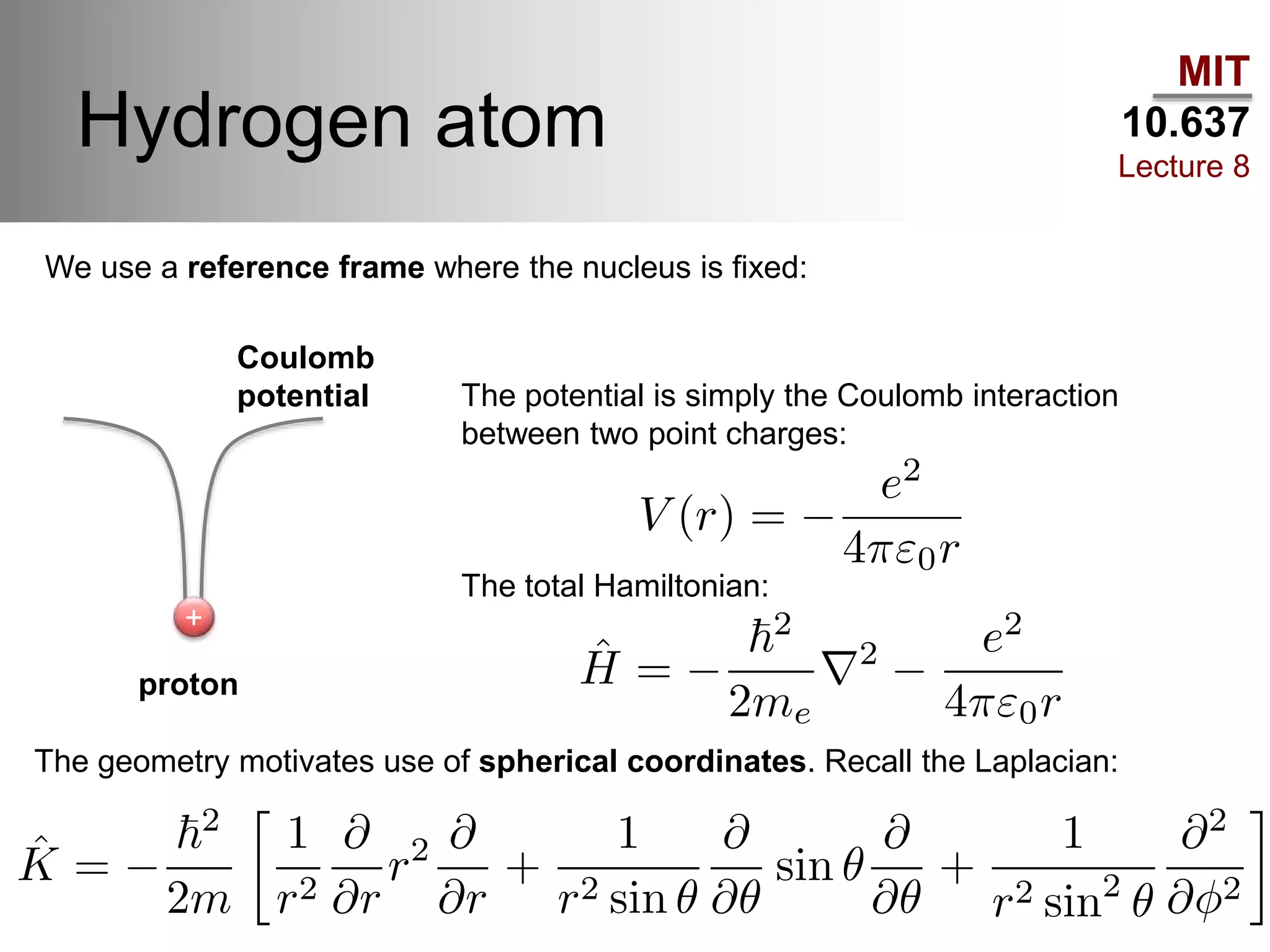
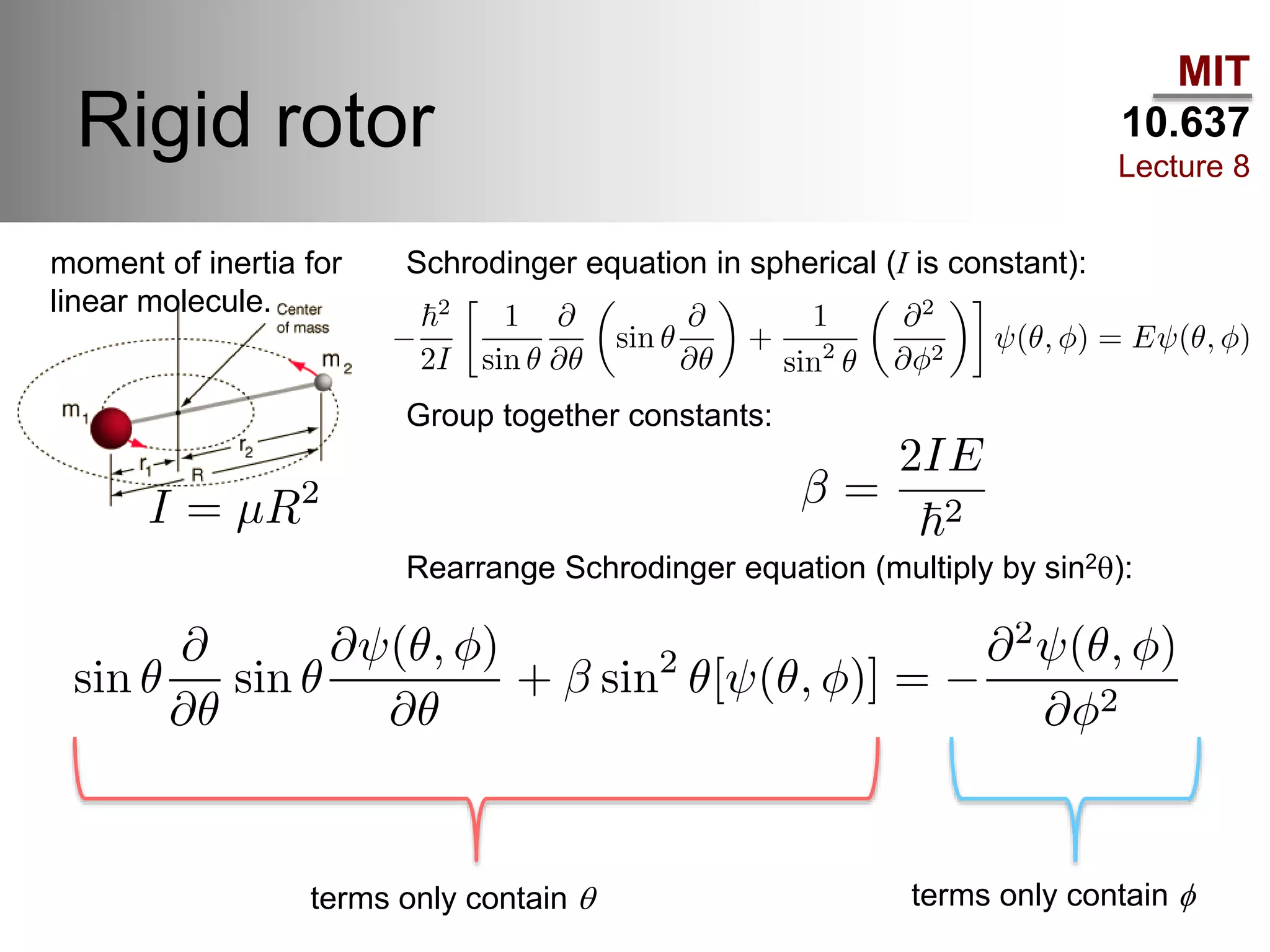
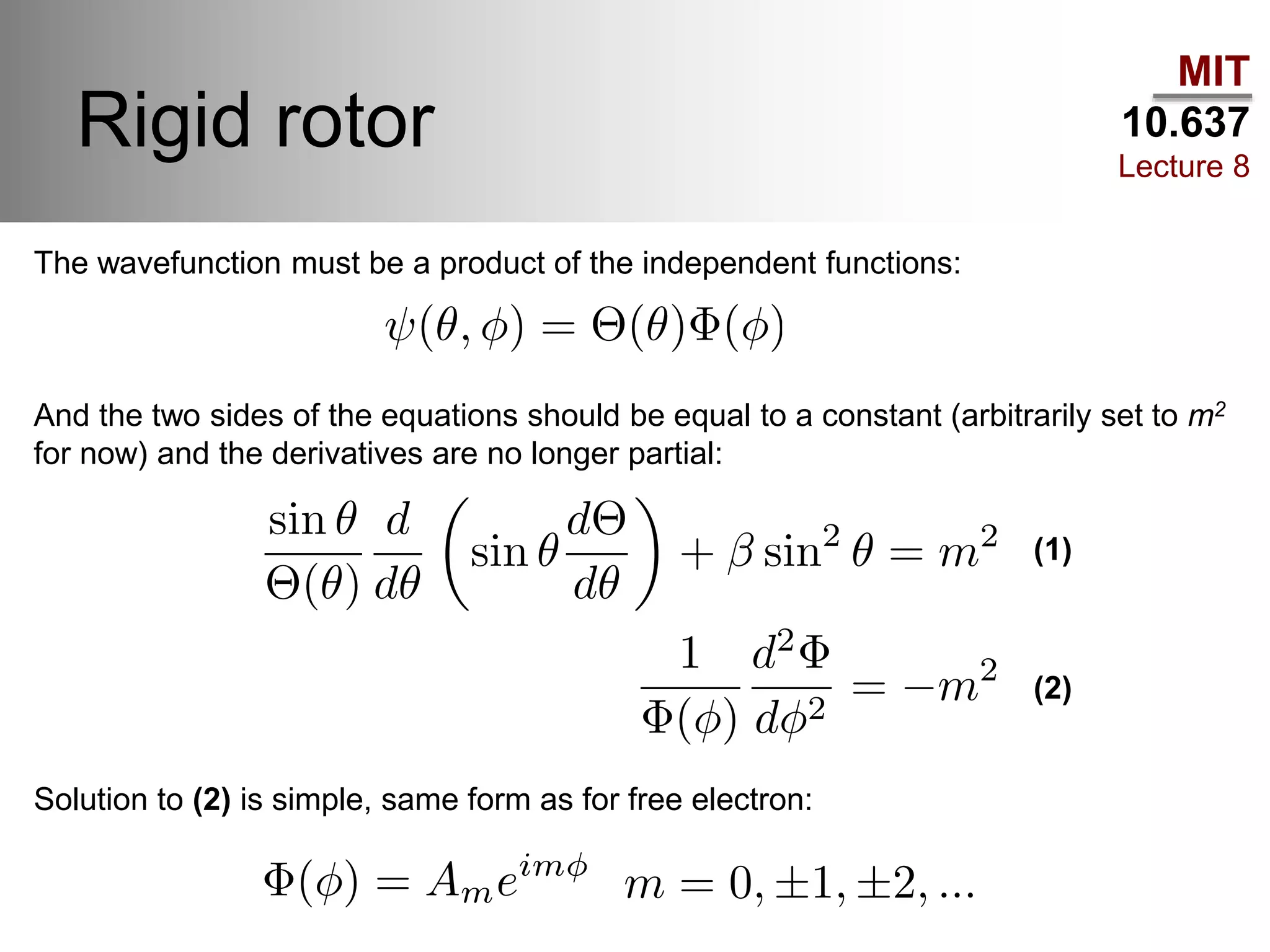
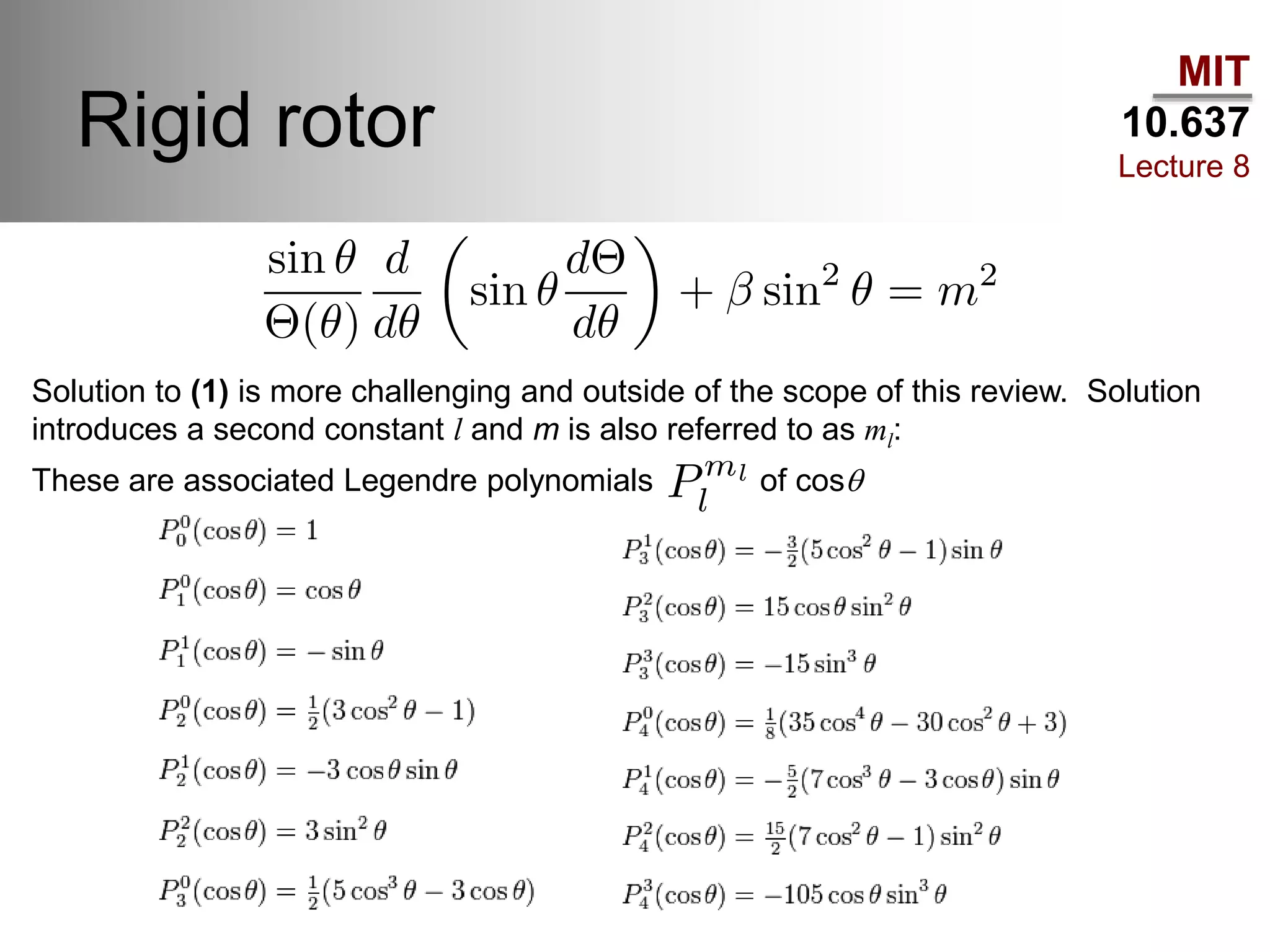
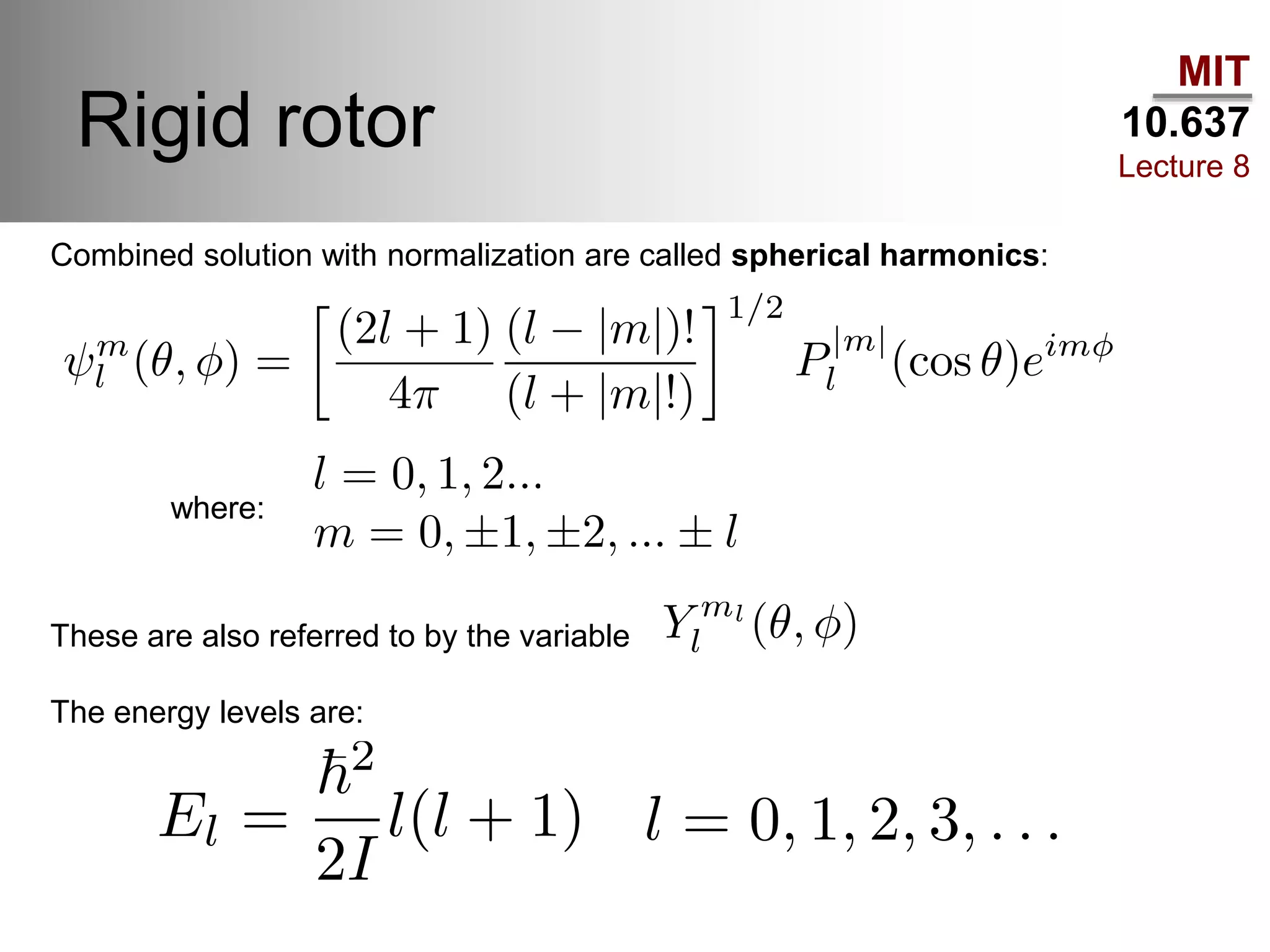
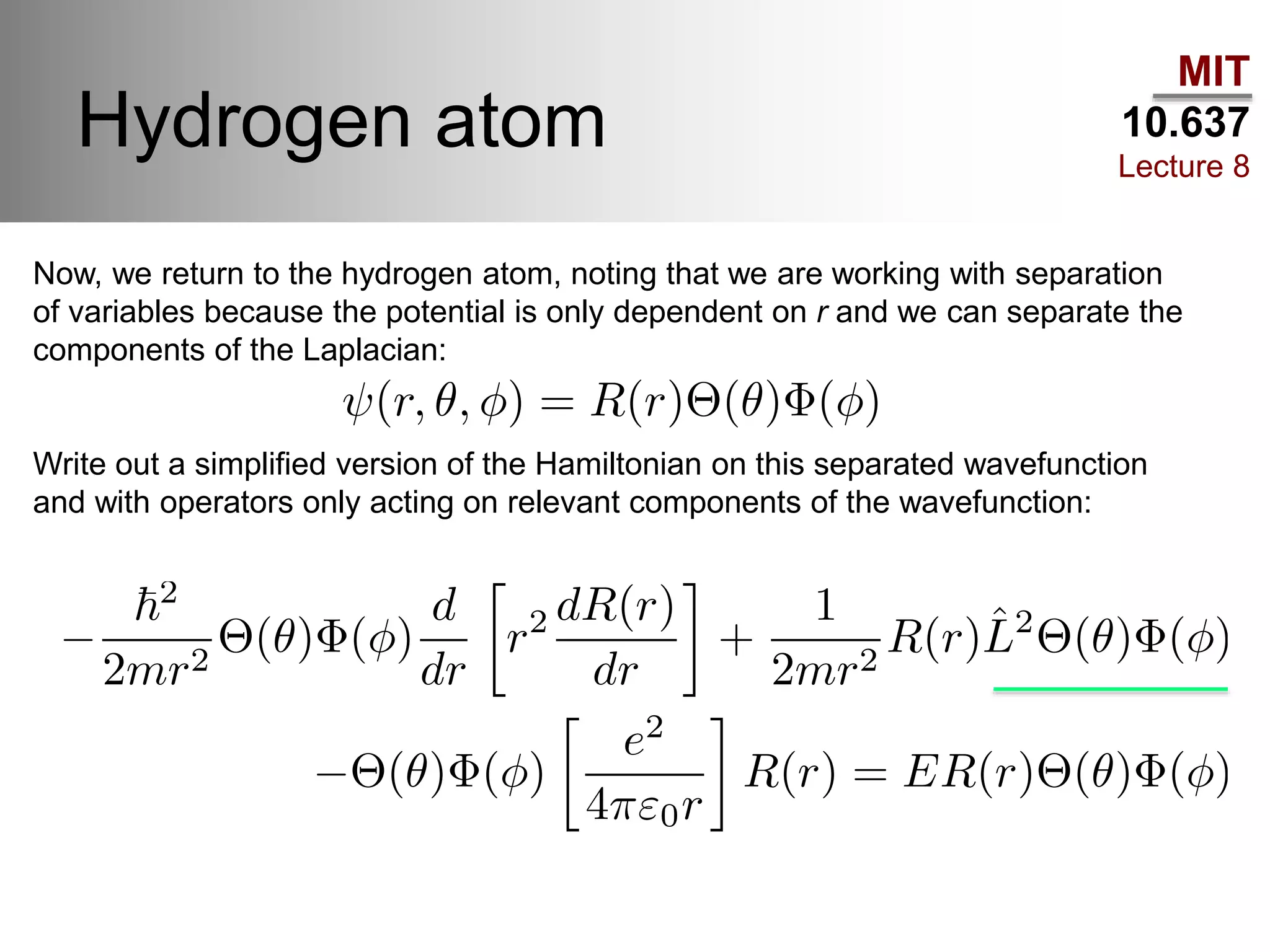
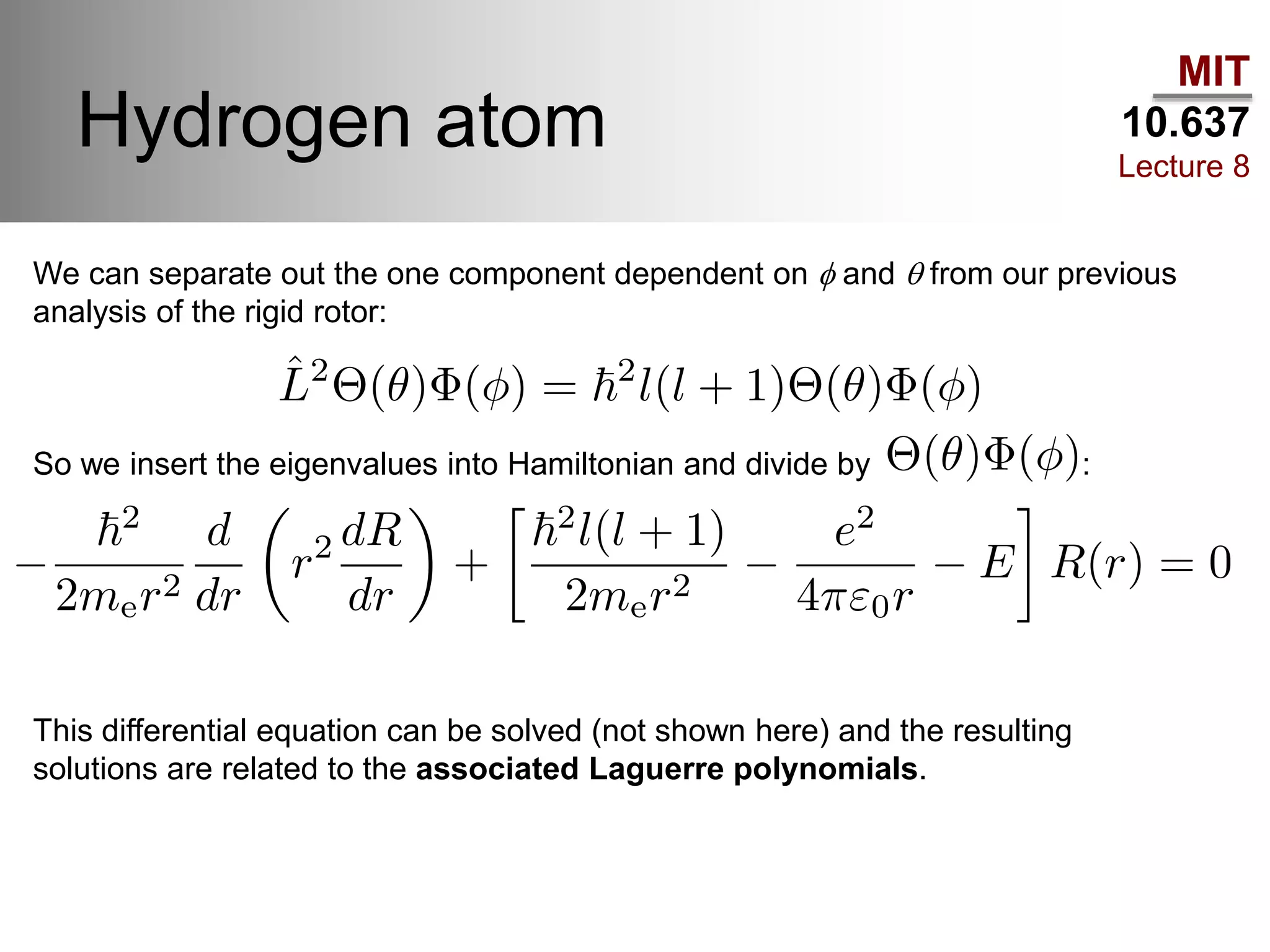
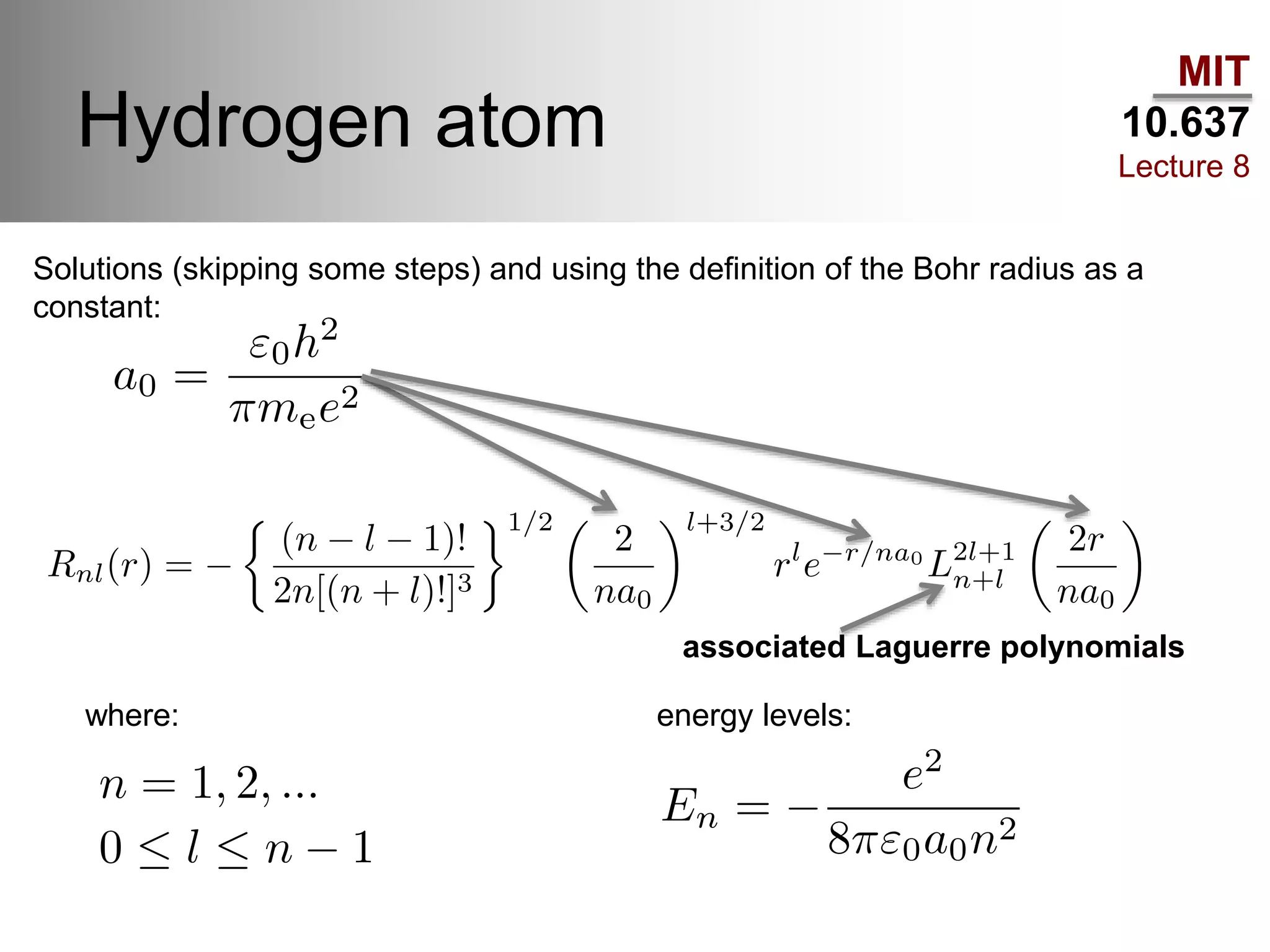
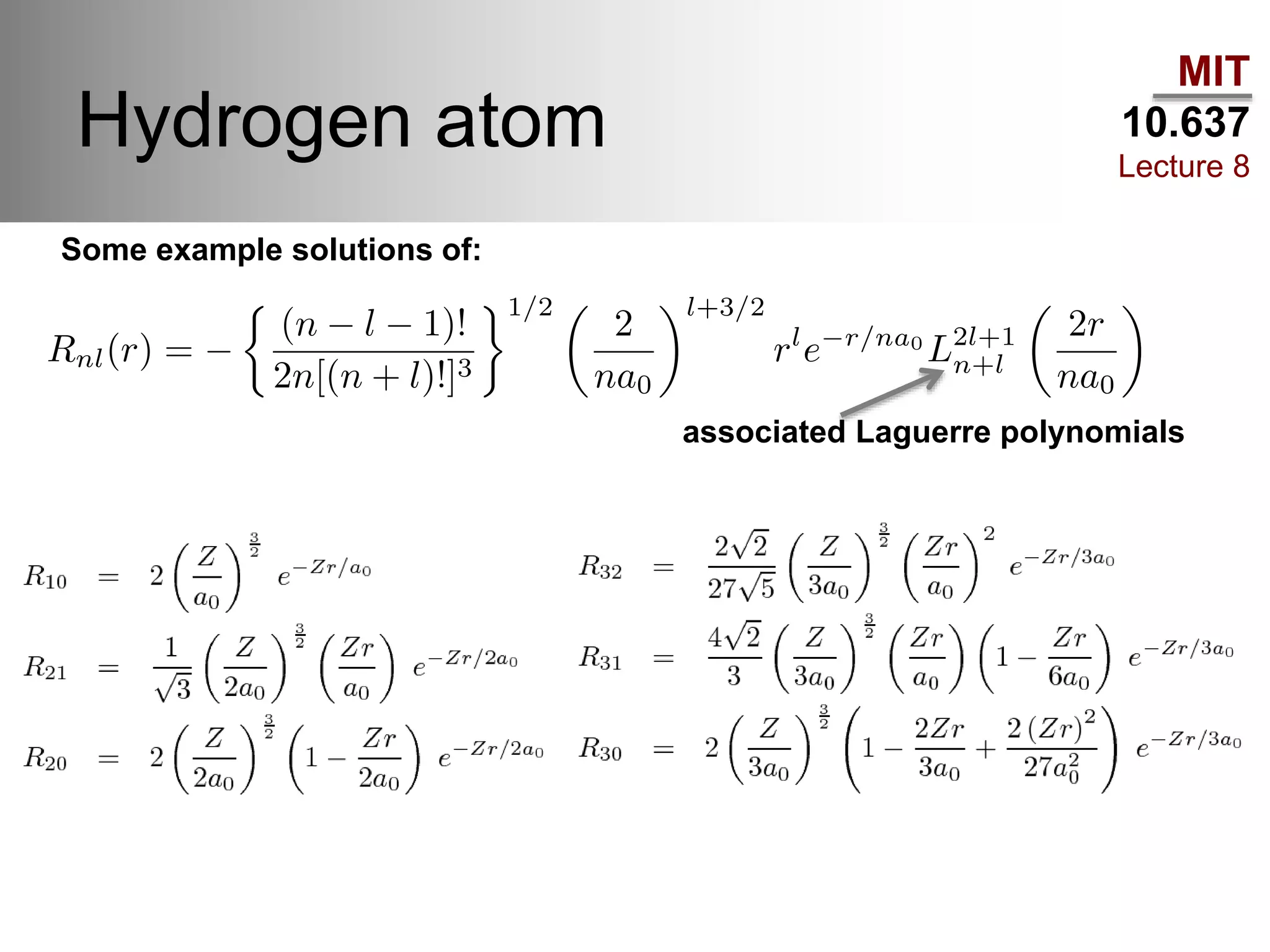
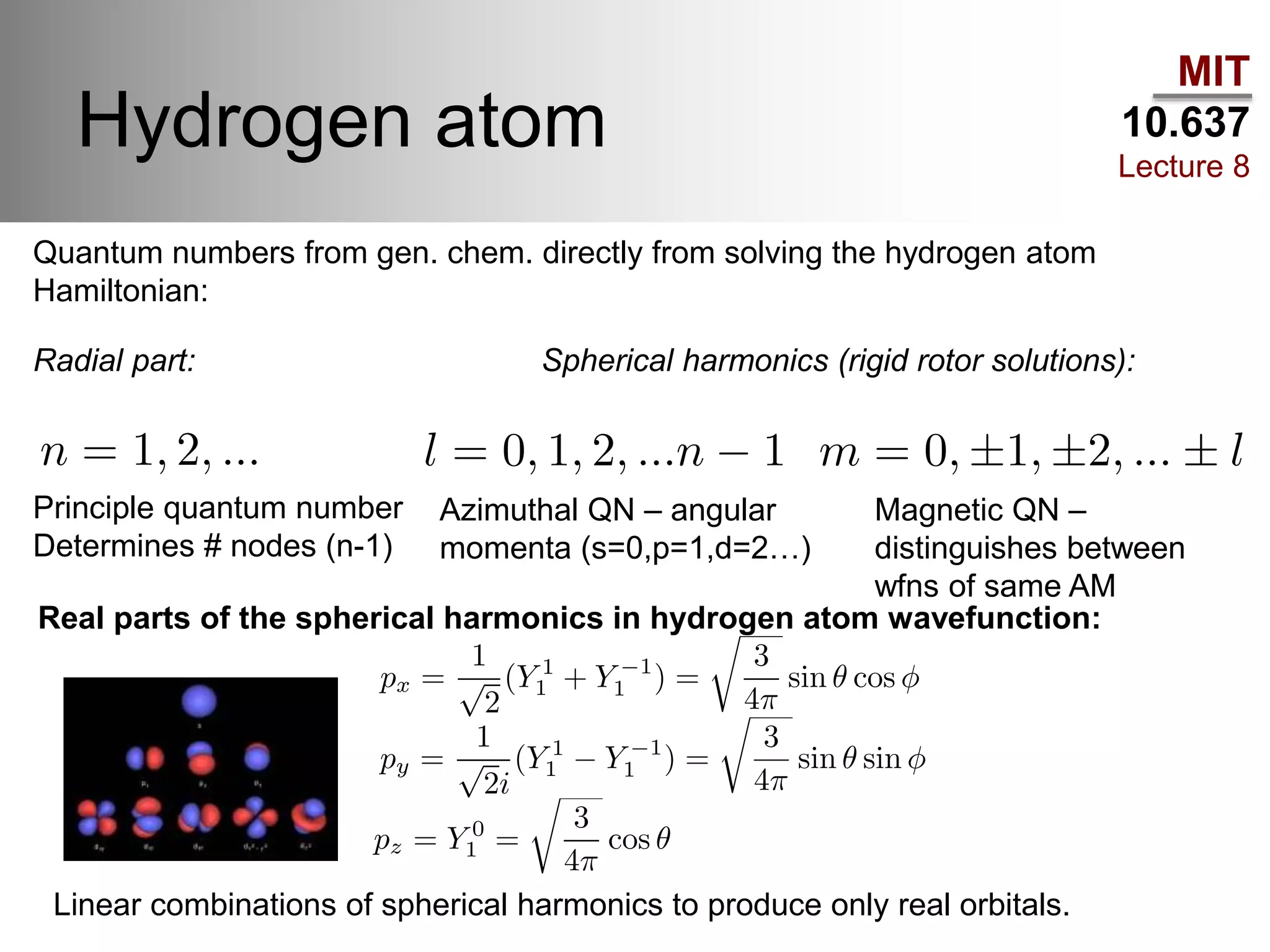
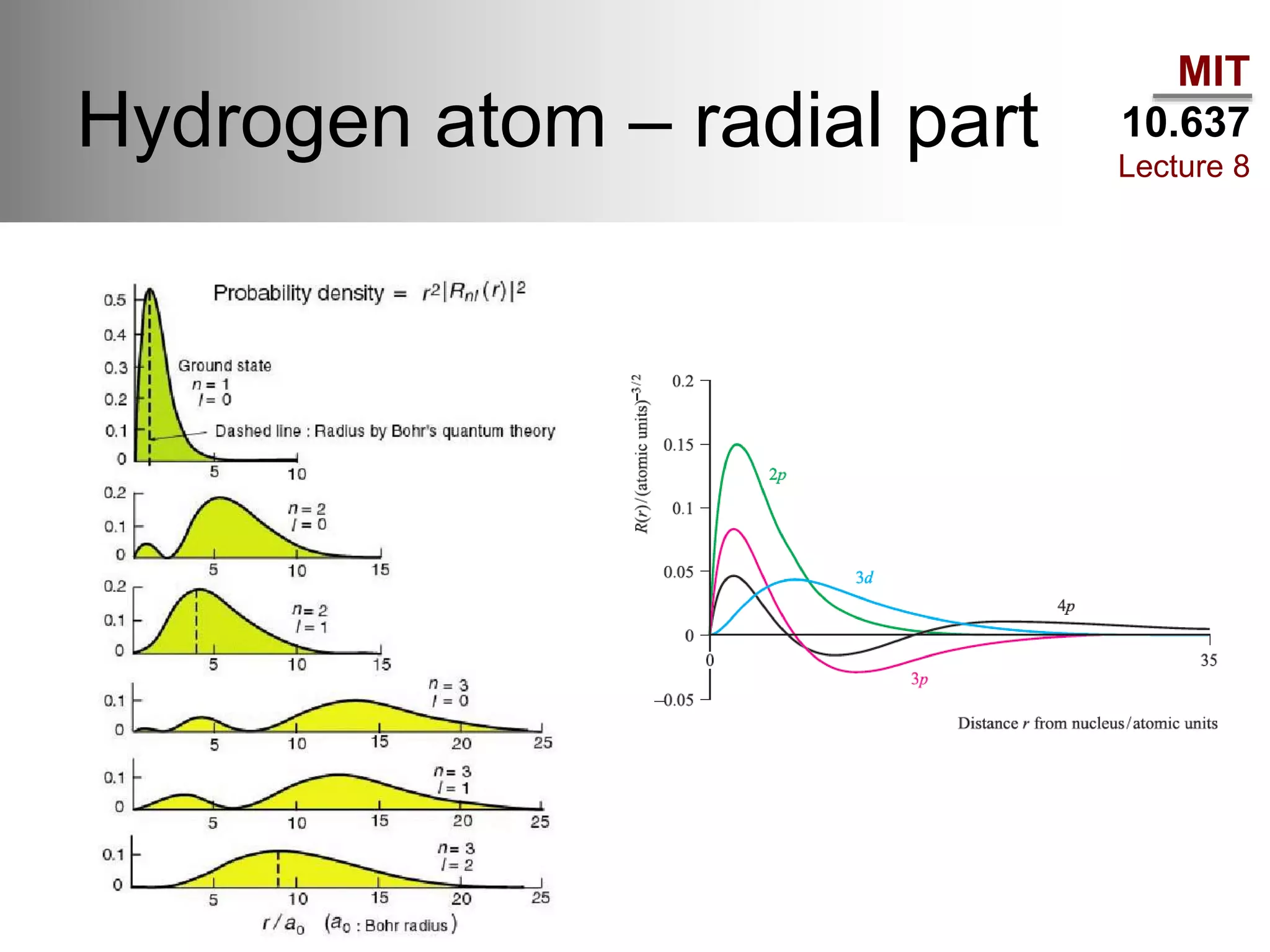
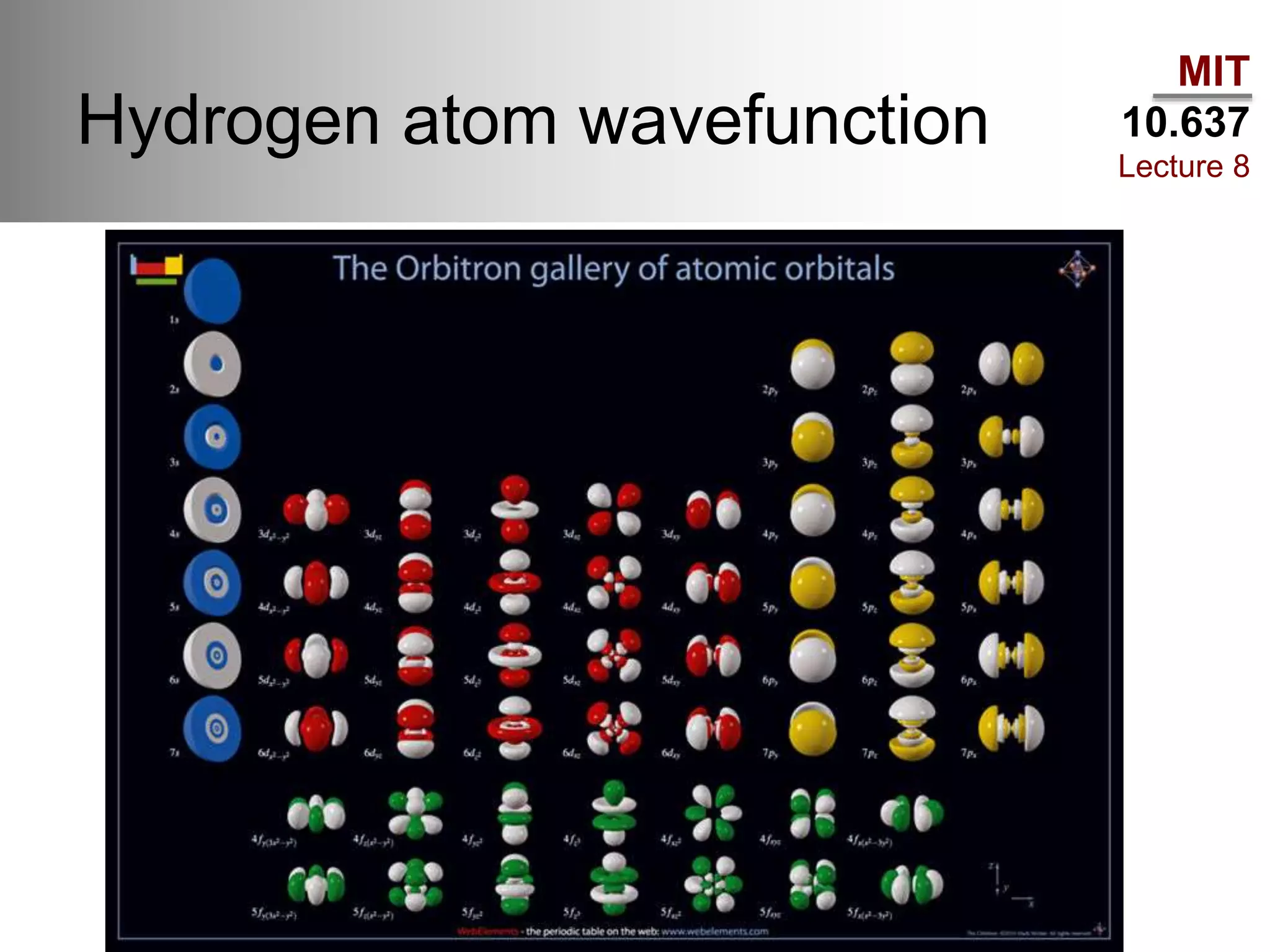
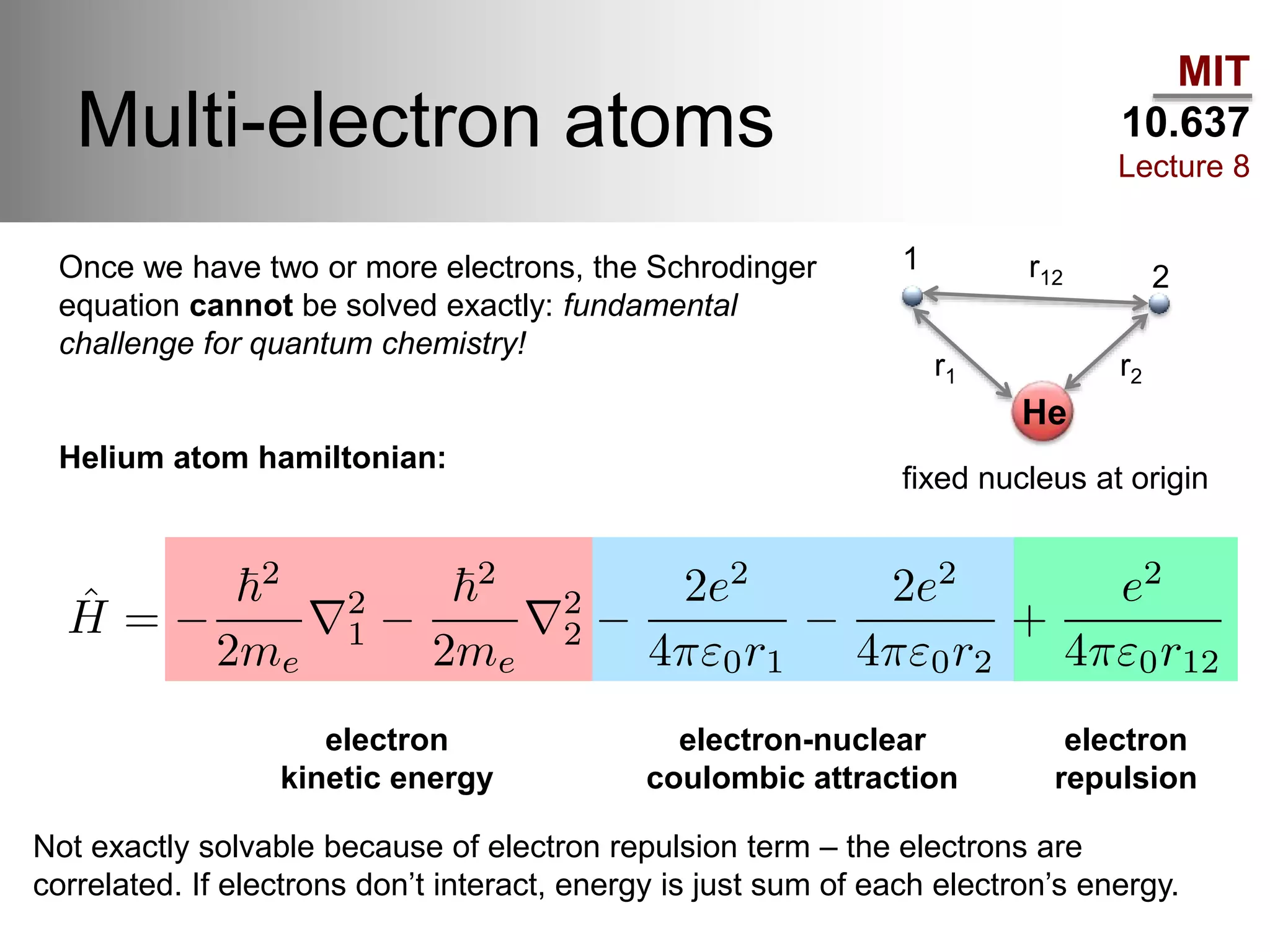
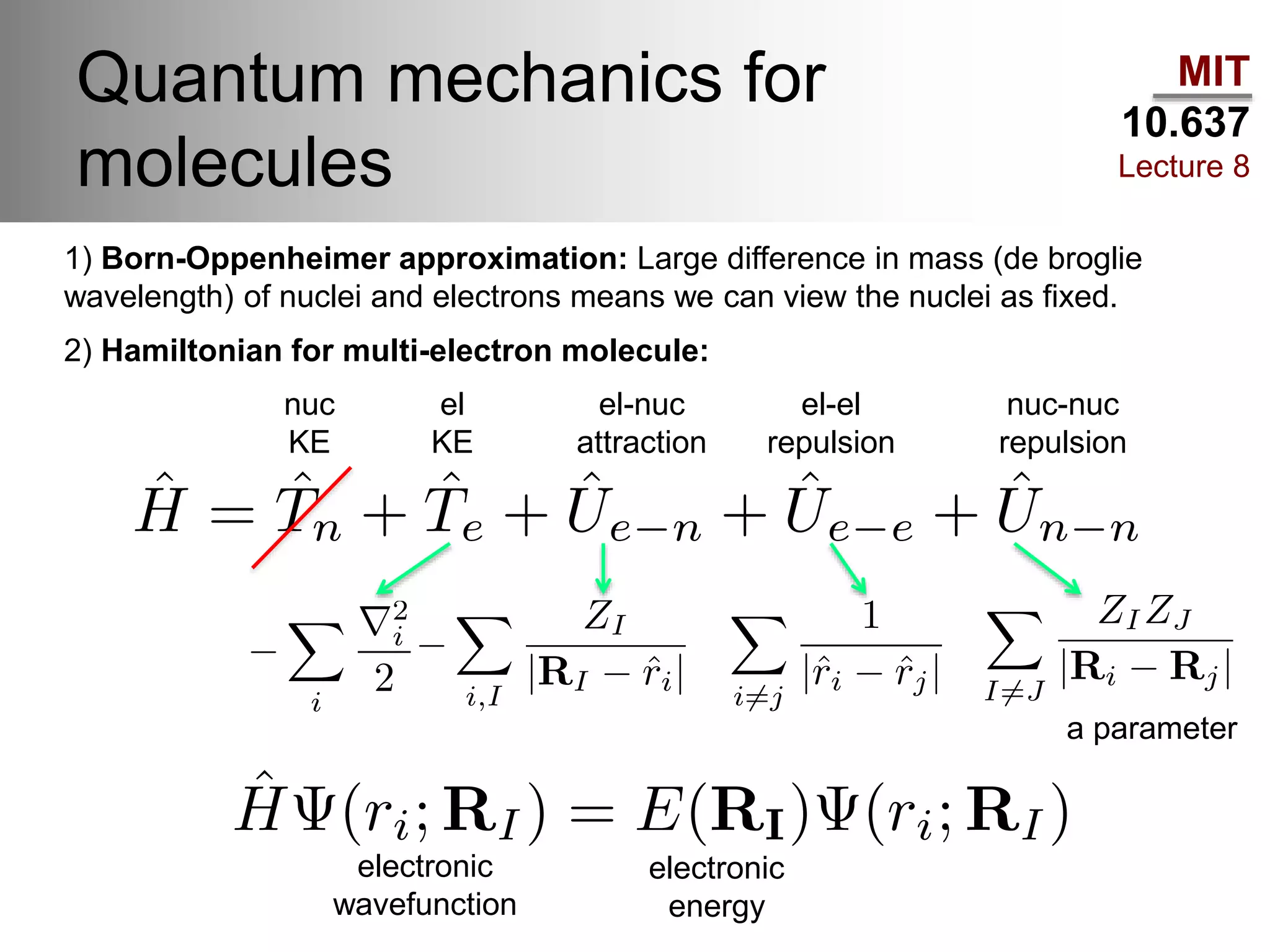
This lecture discusses fundamental concepts in quantum mechanics, including black body radiation, wave-particle duality, and Heisenberg's uncertainty principle. It covers the postulates of quantum mechanics, properties of operators, and solutions to the Schrödinger equation for systems like the hydrogen atom and multi-electron atoms. The document highlights key theories, mathematical formulations, and the use of concepts like the Born-Oppenheimer approximation and perturbation theory in quantum chemistry.