Tang 02 alcohols, aldehydes, ketones 2
•
4 likes•1,104 views
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
IUPAC Nomenclature_Pharmaceutical Organic Chemistry

IUPAC Nomenclature_Pharmaceutical Organic Chemistry
IUPAC NOMENCLATURE_ORGANIC_for JEE(MAIN)-JEE(ADVANCED)-NEET

IUPAC NOMENCLATURE_ORGANIC_for JEE(MAIN)-JEE(ADVANCED)-NEET
NOMENCLATURE OF ORGANIC COMPOUNDS BY -- KHUSH AHUJA

NOMENCLATURE OF ORGANIC COMPOUNDS BY -- KHUSH AHUJA
Organic Chemistry: Classification of Organic Compounds: Seminar

Organic Chemistry: Classification of Organic Compounds: Seminar
Similar to Tang 02 alcohols, aldehydes, ketones 2
Similar to Tang 02 alcohols, aldehydes, ketones 2 (20)
IUPAC Nomenclature of Organic Compounds Part-2.pptx

IUPAC Nomenclature of Organic Compounds Part-2.pptx
More from mrtangextrahelp
More from mrtangextrahelp (20)
Recently uploaded
Recently uploaded (20)
Navigating the Deluge_ Dubai Floods and the Resilience of Dubai International...

Navigating the Deluge_ Dubai Floods and the Resilience of Dubai International...
2024: Domino Containers - The Next Step. News from the Domino Container commu...

2024: Domino Containers - The Next Step. News from the Domino Container commu...
Apidays New York 2024 - Accelerating FinTech Innovation by Vasa Krishnan, Fin...

Apidays New York 2024 - Accelerating FinTech Innovation by Vasa Krishnan, Fin...
CNIC Information System with Pakdata Cf In Pakistan

CNIC Information System with Pakdata Cf In Pakistan
Repurposing LNG terminals for Hydrogen Ammonia: Feasibility and Cost Saving

Repurposing LNG terminals for Hydrogen Ammonia: Feasibility and Cost Saving
Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe

Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe
Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood

Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood
"I see eyes in my soup": How Delivery Hero implemented the safety system for ...

"I see eyes in my soup": How Delivery Hero implemented the safety system for ...
Web Form Automation for Bonterra Impact Management (fka Social Solutions Apri...

Web Form Automation for Bonterra Impact Management (fka Social Solutions Apri...
Axa Assurance Maroc - Insurer Innovation Award 2024

Axa Assurance Maroc - Insurer Innovation Award 2024
Biography Of Angeliki Cooney | Senior Vice President Life Sciences | Albany, ...

Biography Of Angeliki Cooney | Senior Vice President Life Sciences | Albany, ...
Finding Java's Hidden Performance Traps @ DevoxxUK 2024

Finding Java's Hidden Performance Traps @ DevoxxUK 2024
Apidays New York 2024 - APIs in 2030: The Risk of Technological Sleepwalk by ...

Apidays New York 2024 - APIs in 2030: The Risk of Technological Sleepwalk by ...
TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery

TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery
Tang 02 alcohols, aldehydes, ketones 2
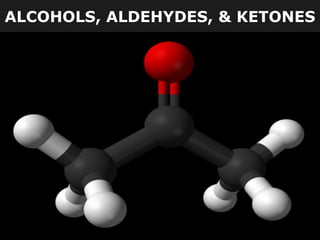
- 1. ALCOHOLS, ALDEHYDES, & KETONES
- 2. ALCOHOLS, ALDEHYDES, & KETONES
- 12. Other Nomenclature Prefixes ALDEHYDES # of carbons IUPAC Common System 1 meth- form- 2 eth- acet- 3 prop- proprion- 4 but- butyr-