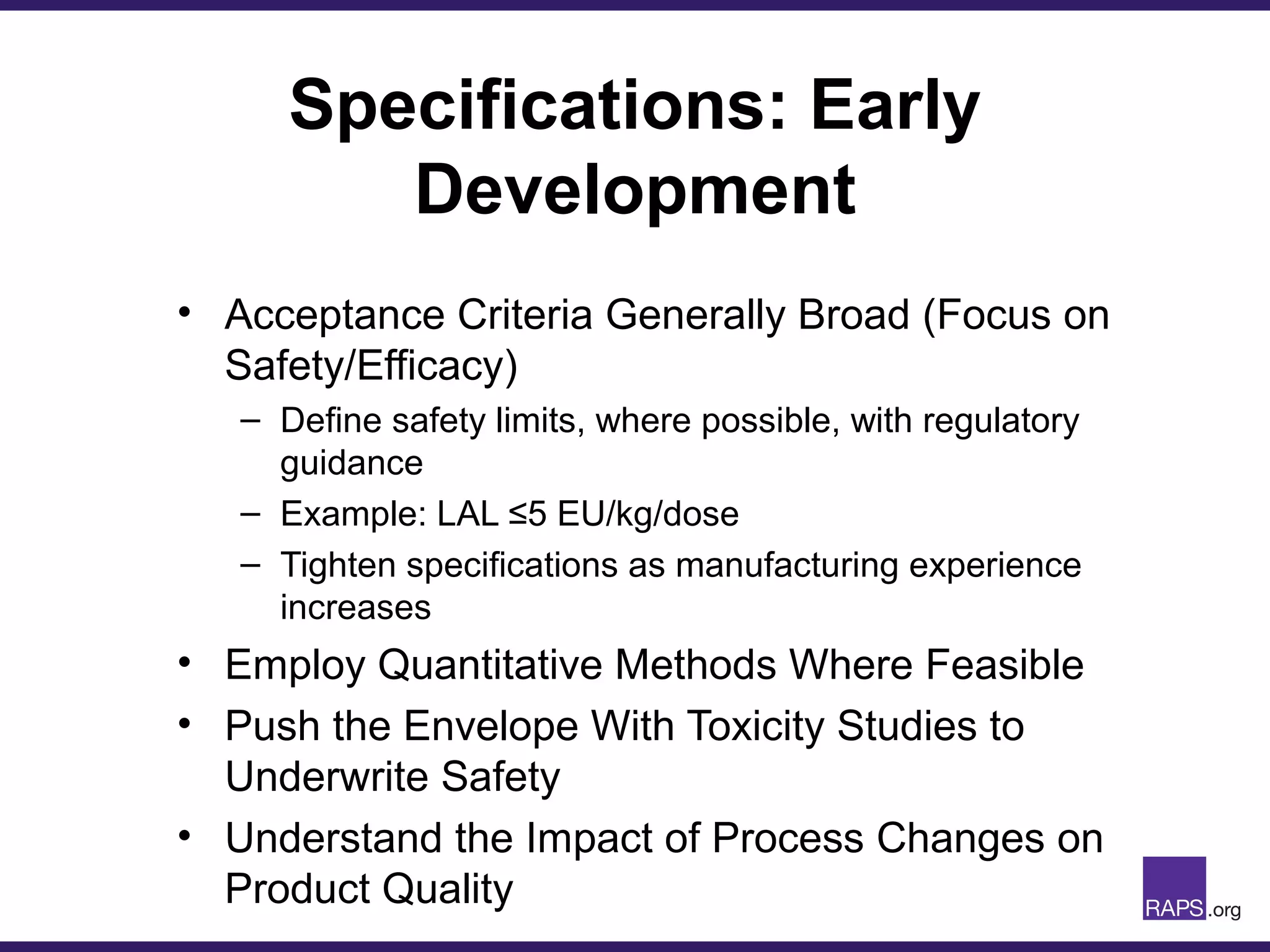
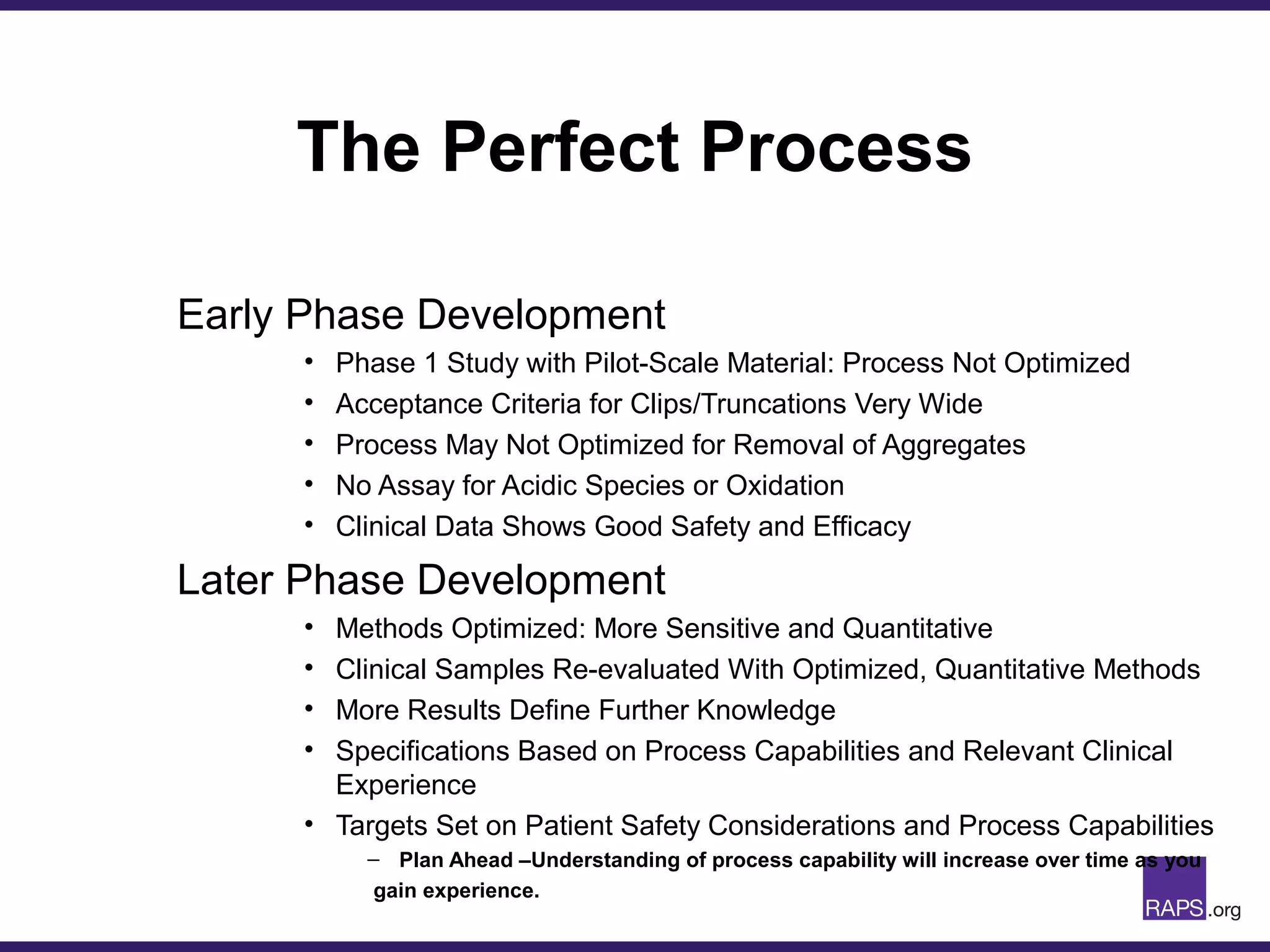
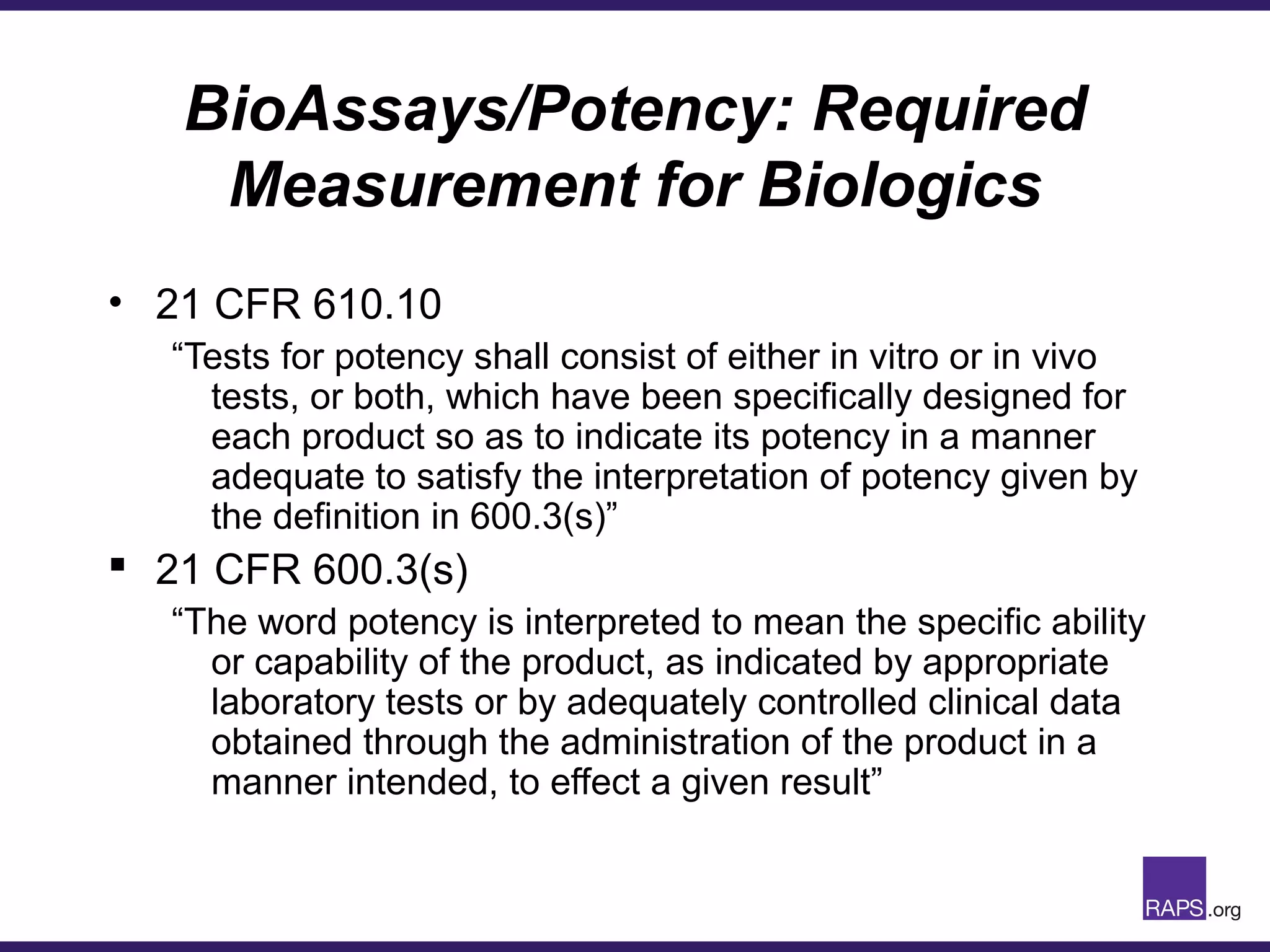
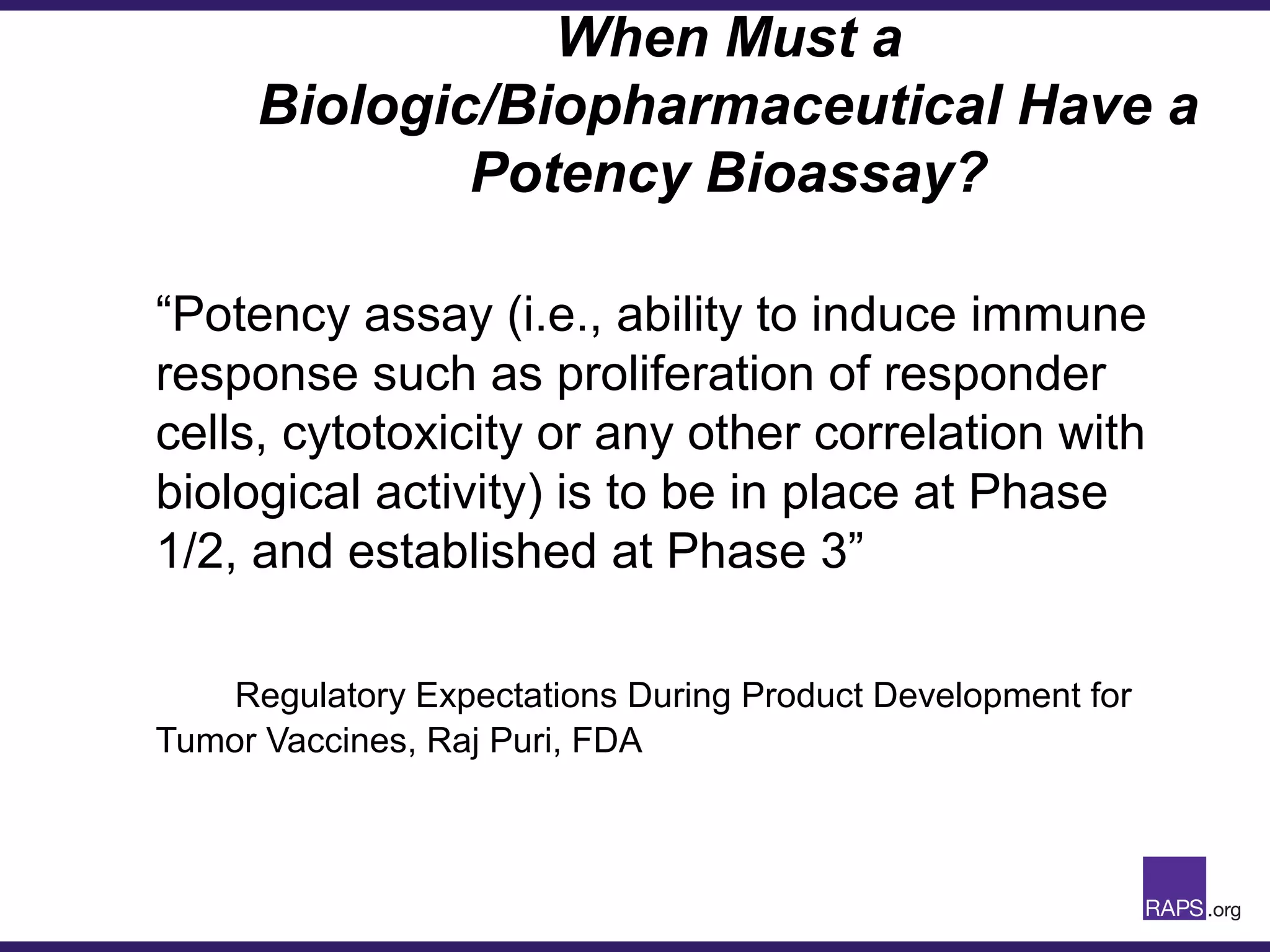
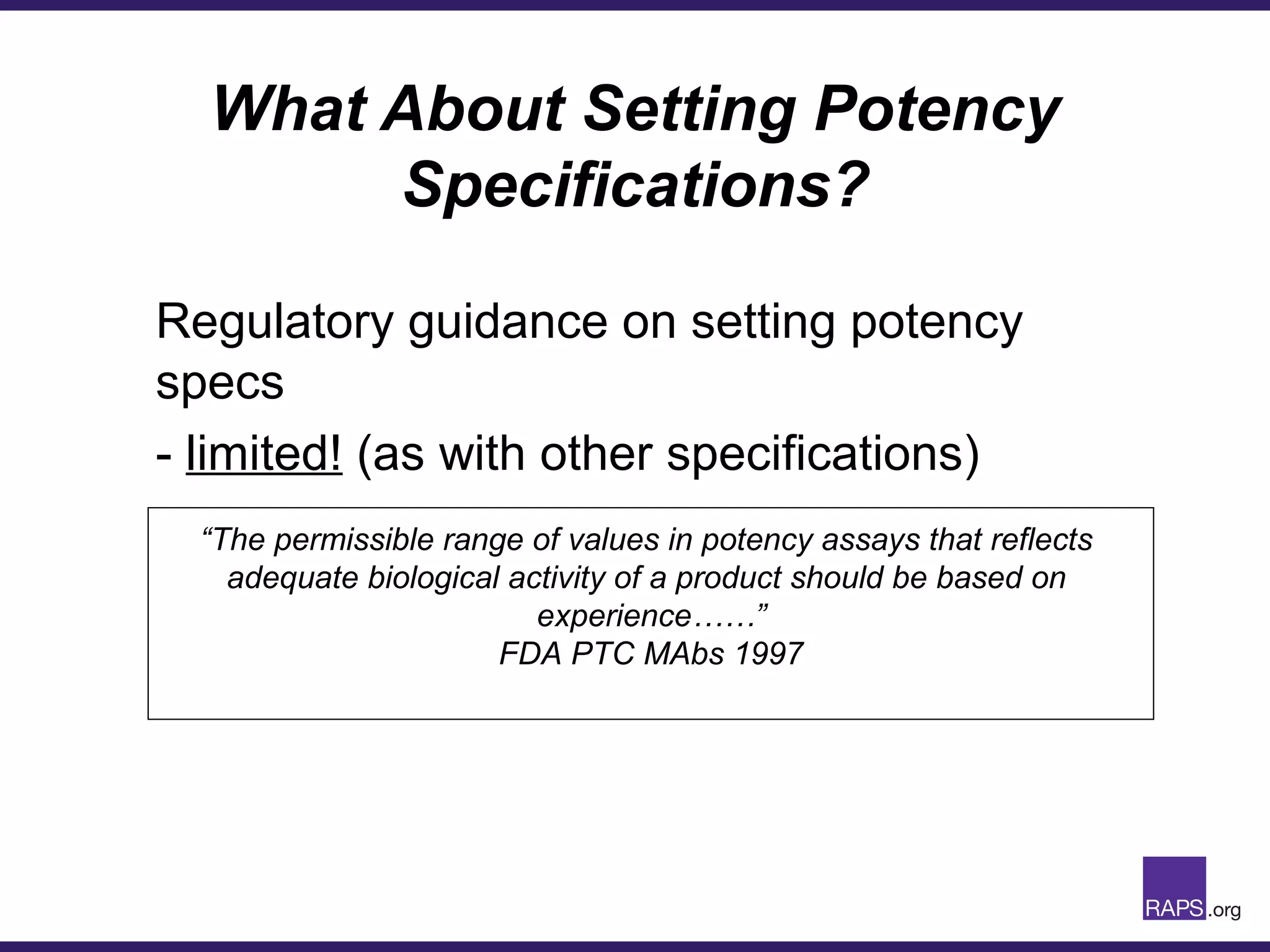
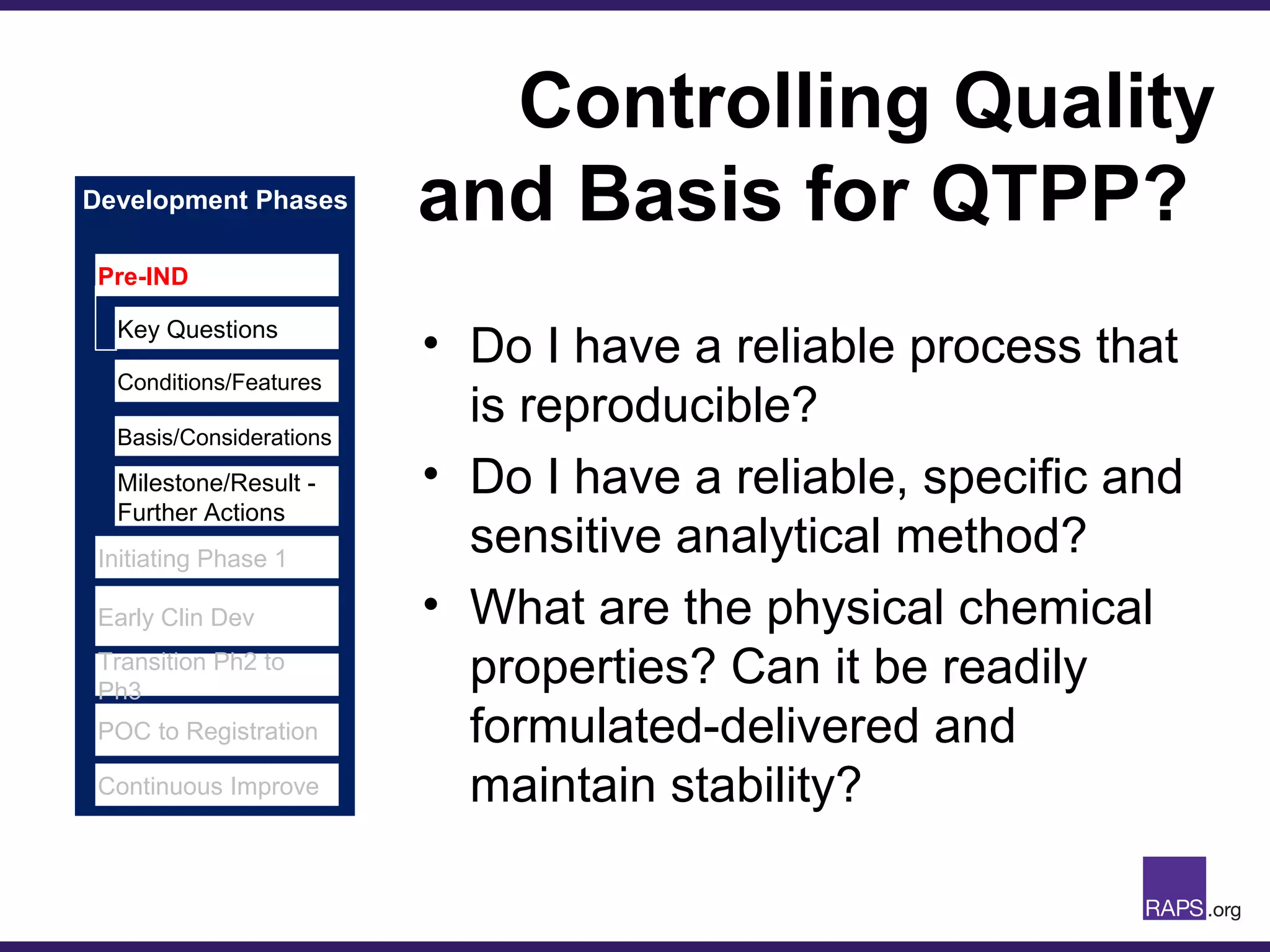
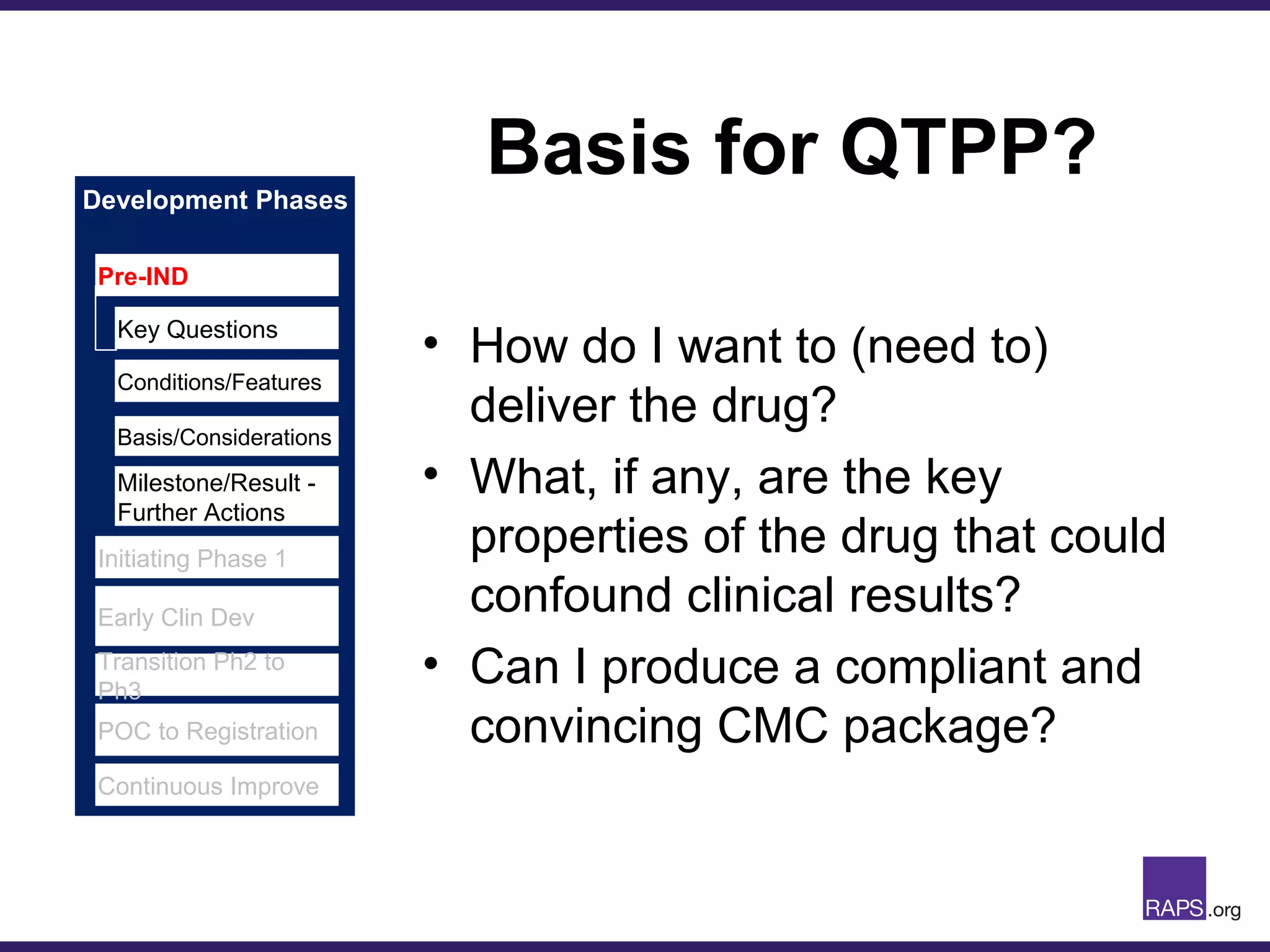
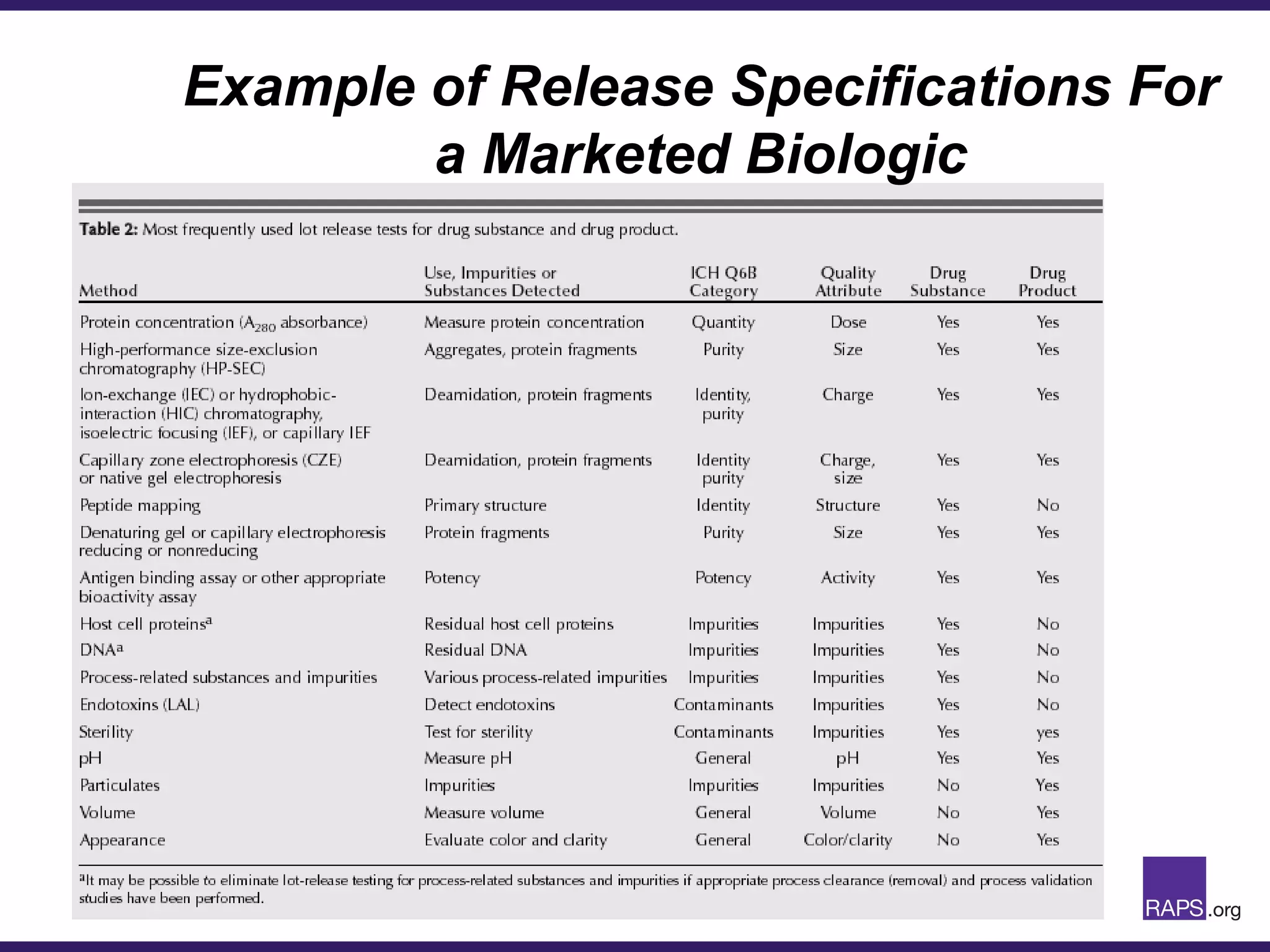
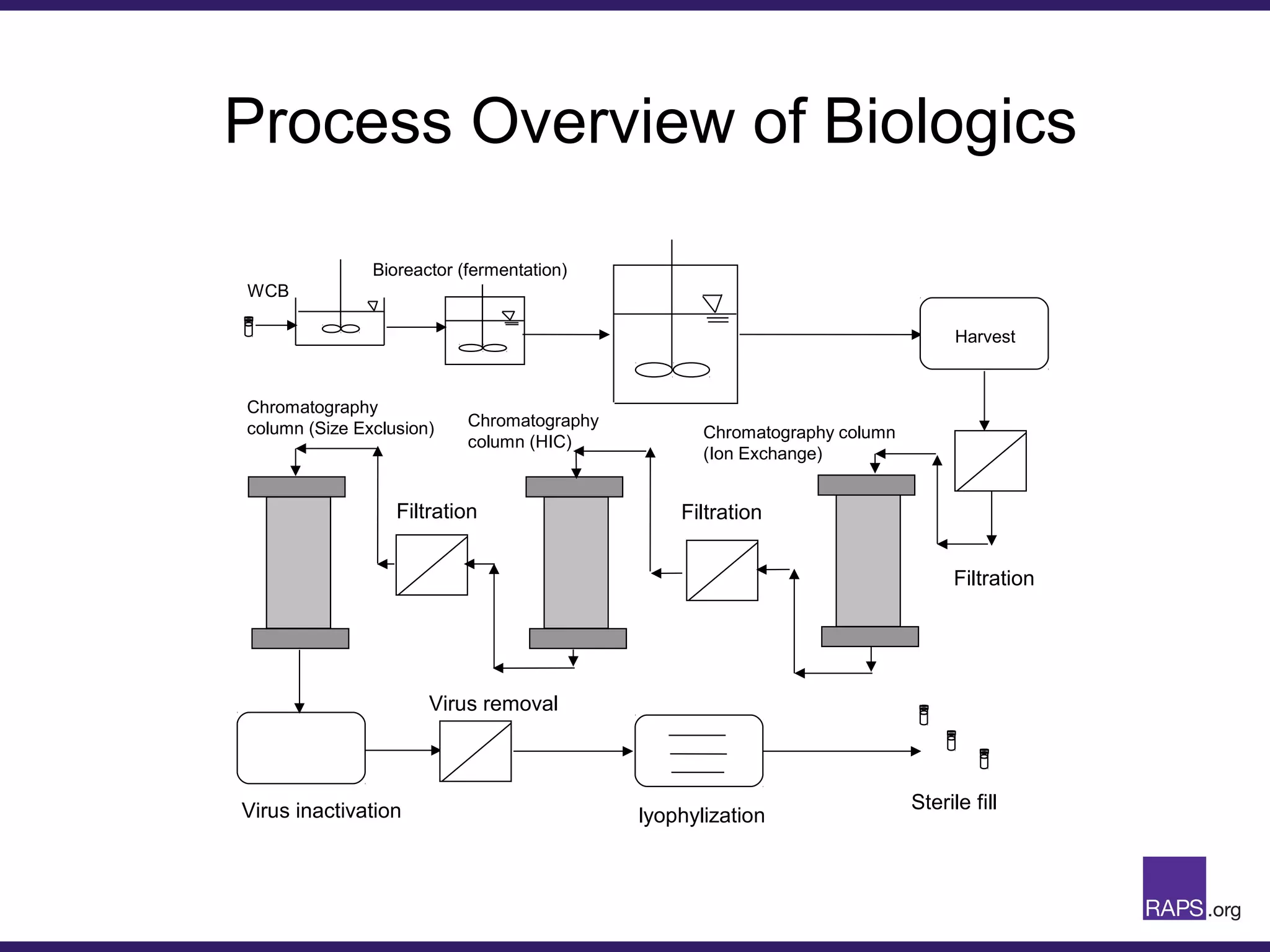
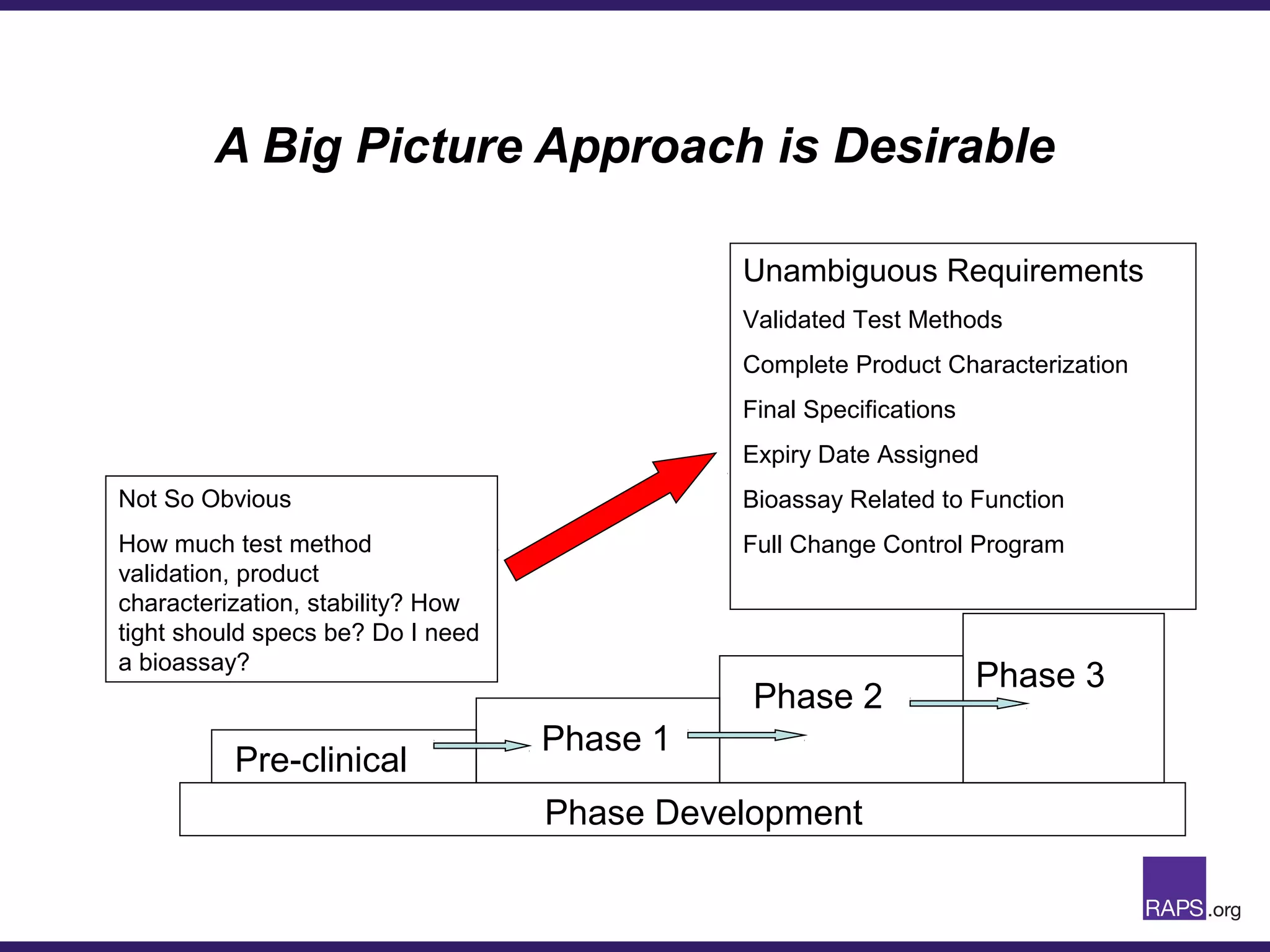
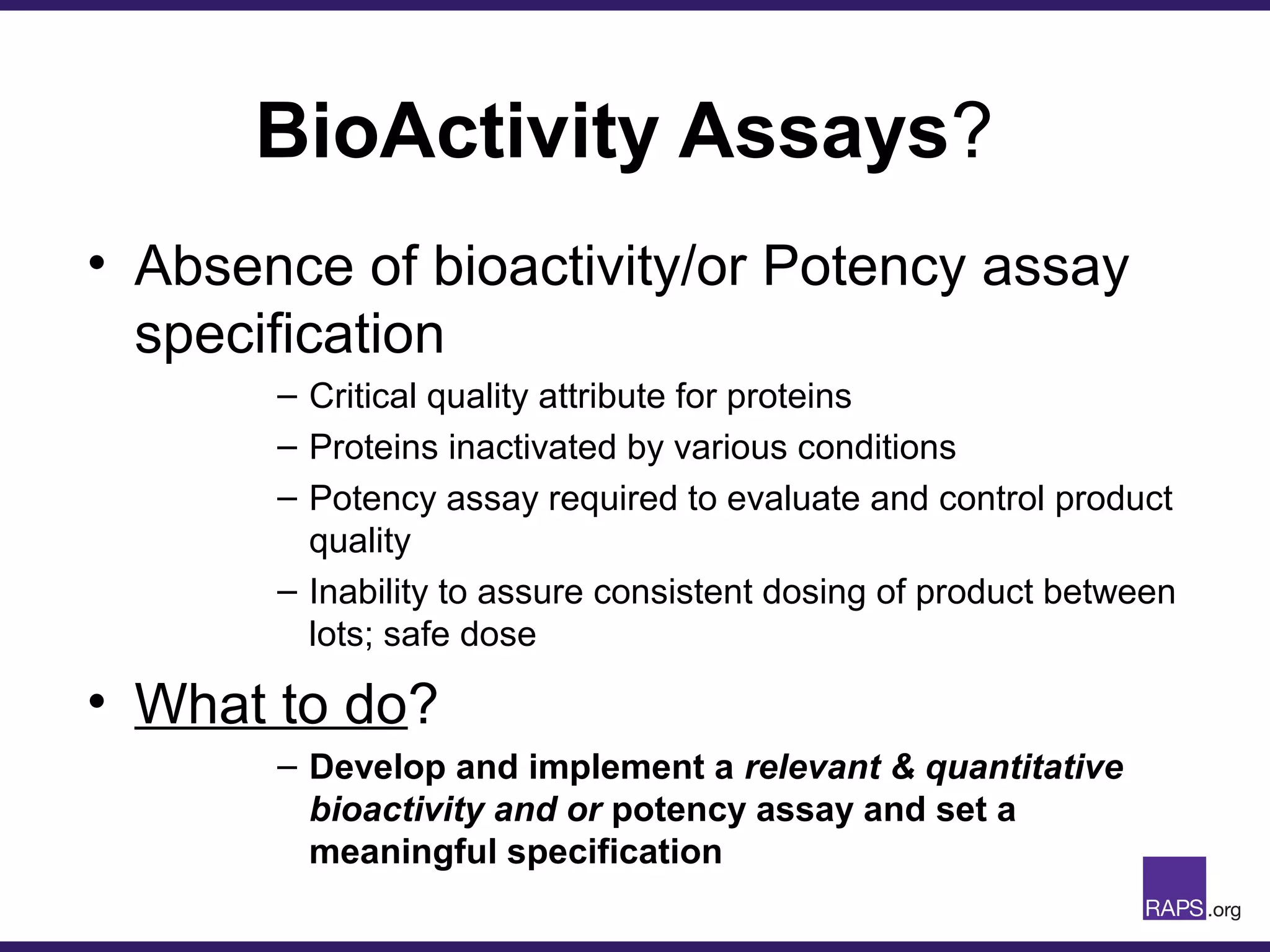
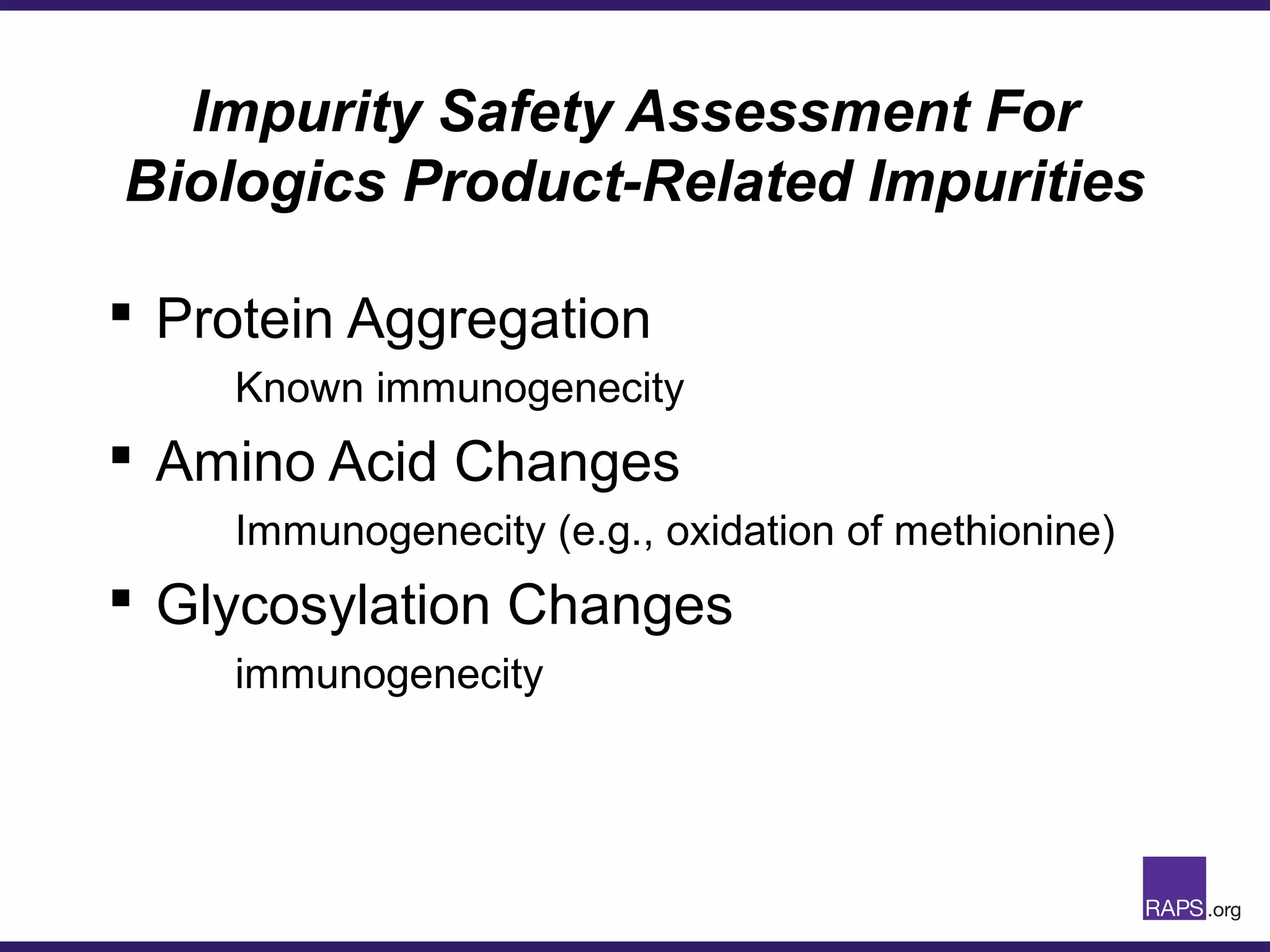
Edward Narke discussed the CMC pathway for biologics through clinical development and market approval. The goals are to better understand FDA requirements, visualize a cost-effective approach to manage manufacturing processes, and appreciate challenges in controlling safety, potency, and impurities. Biologics have complex structures that must be characterized and controlled. Assay methods, product specifications, stability data, and comparability between clinical and commercial materials are common reasons INDs are placed on clinical hold. Managing impurities, developing relevant potency assays, and collecting data continuously are important strategies to address these challenges over the course of development.










![List All Actual/Potential Impurities!
• Process-related impurities
Cell-substrate (DNA, HCP, proteases, endotoxins)
Cell-culture (cell-substrate [DNA, HCP, protease];
endotoxin; media components – antibiotics [tetracycline,
gentamicin], hormones [insulin, IGF-1, transferrin],
serum)
Purification (enzymes [DNase/RNase]; resin leachates;
surfactants; residual cleaning agents]
Product-related impurities
FDA Guidance for Review Staff and Sponsors: Gene Therapy 2004](https://image.slidesharecdn.com/cmcbiologicspathwaydraft8-140520175701-phpapp02/75/Cmc-biologics-pathway_draft8-16-2048.jpg)