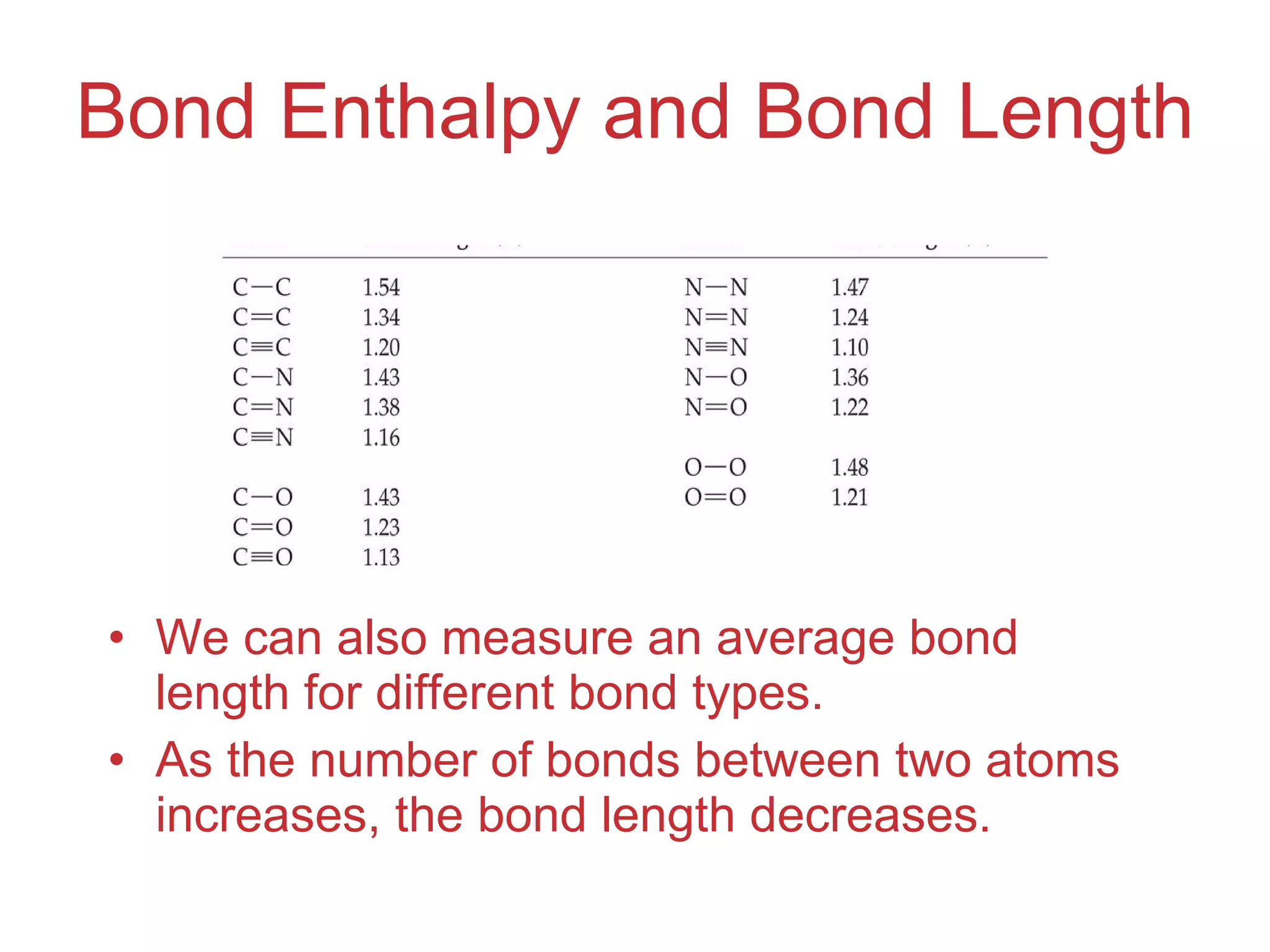
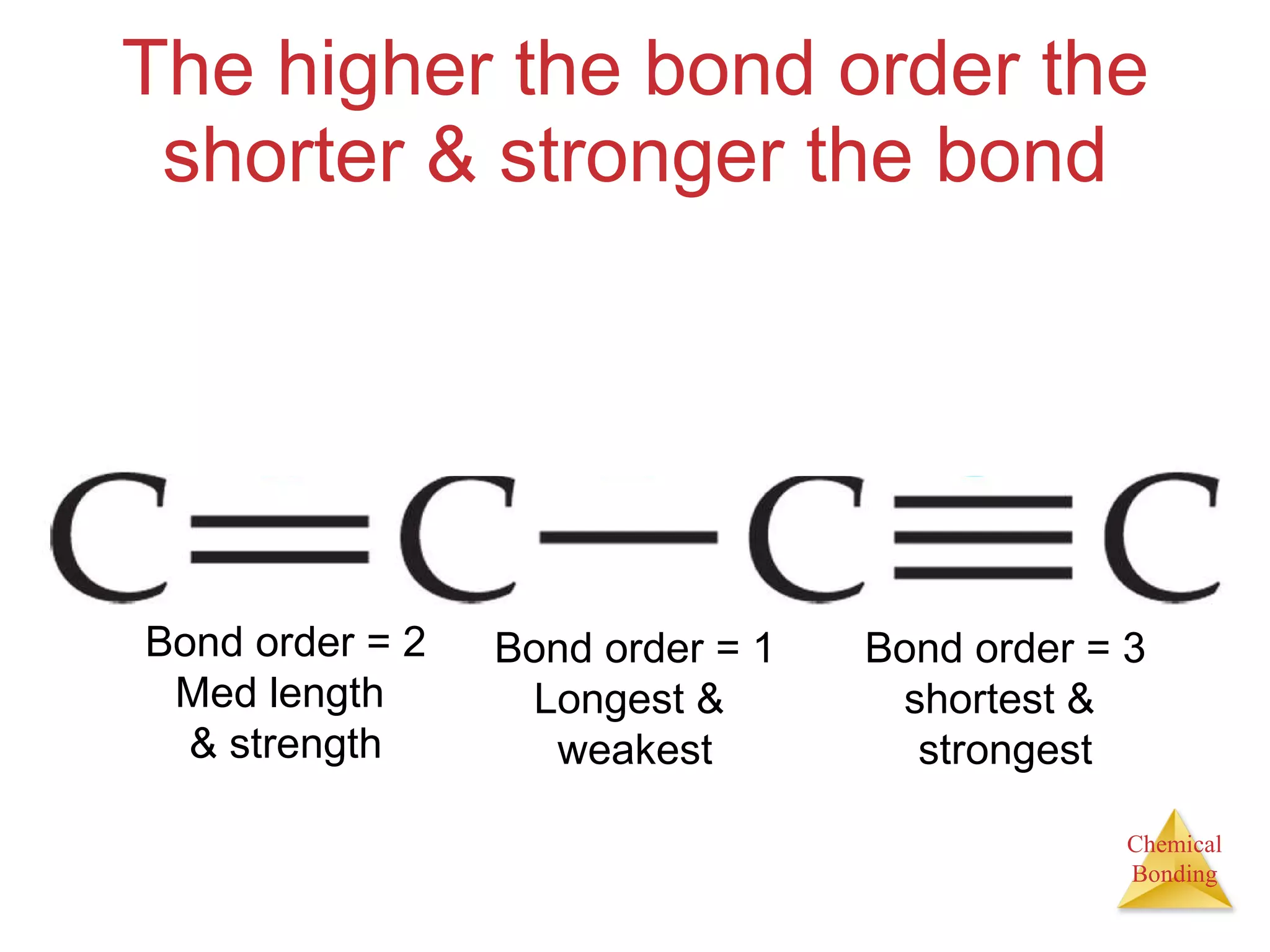
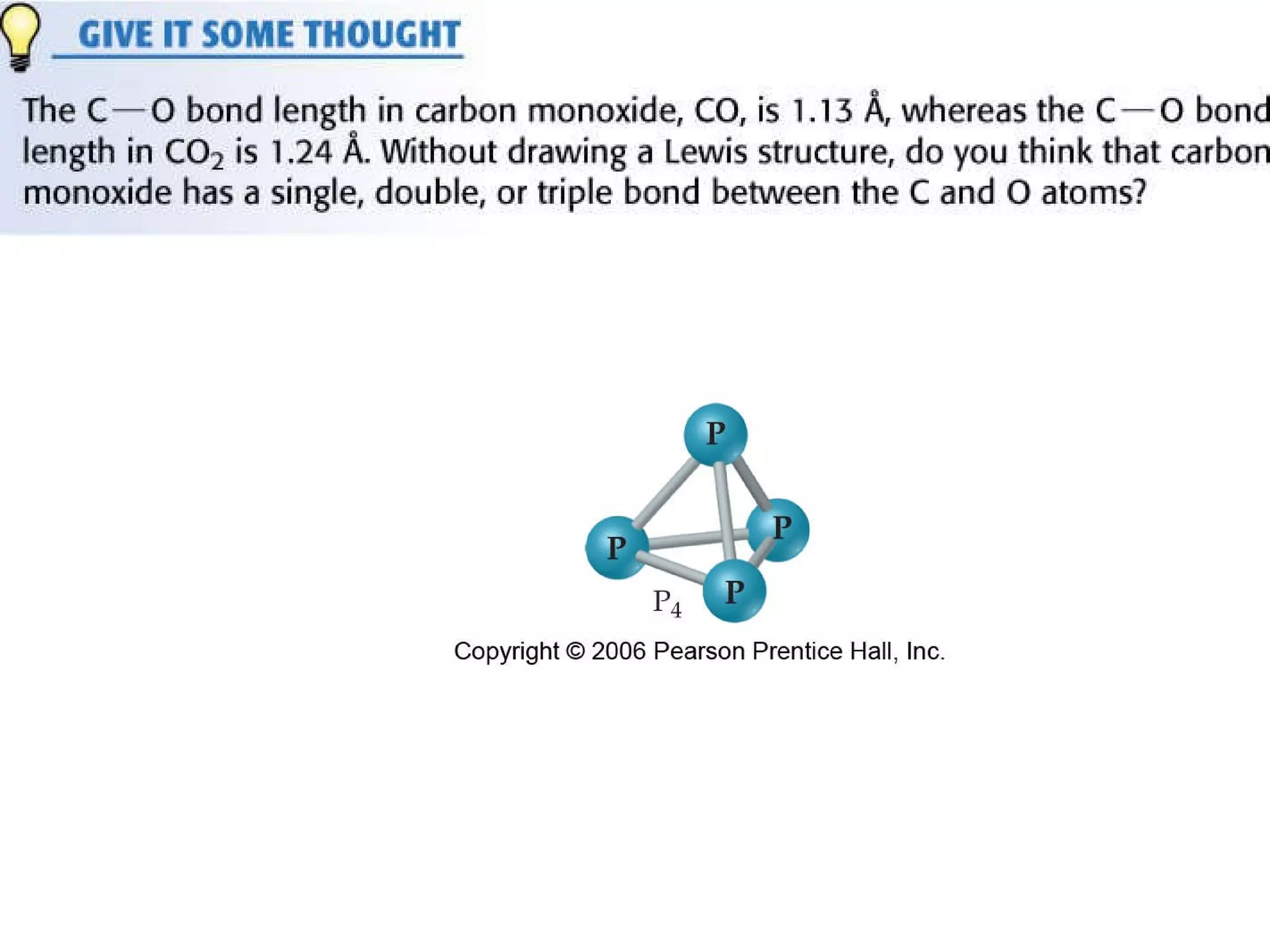
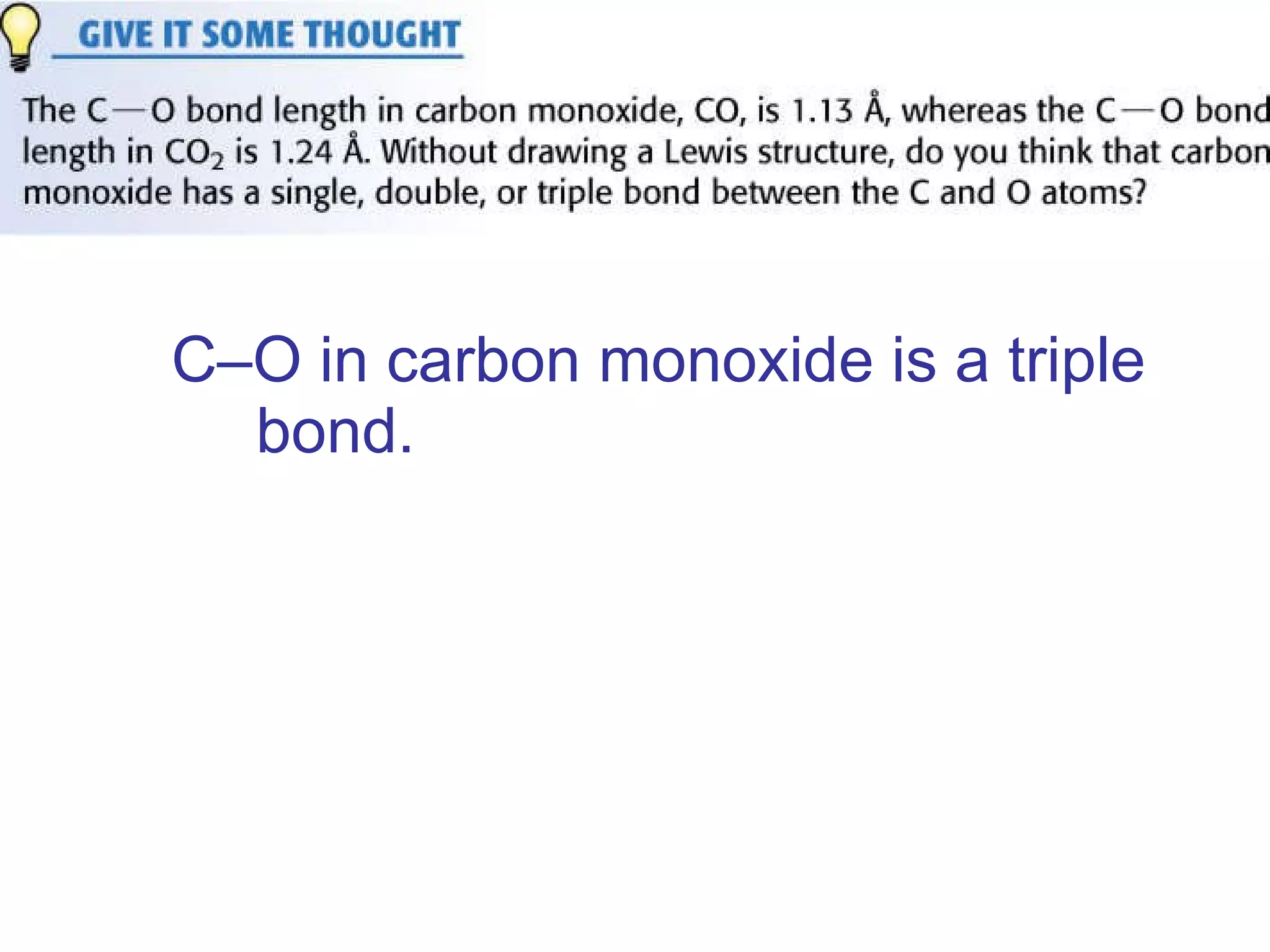
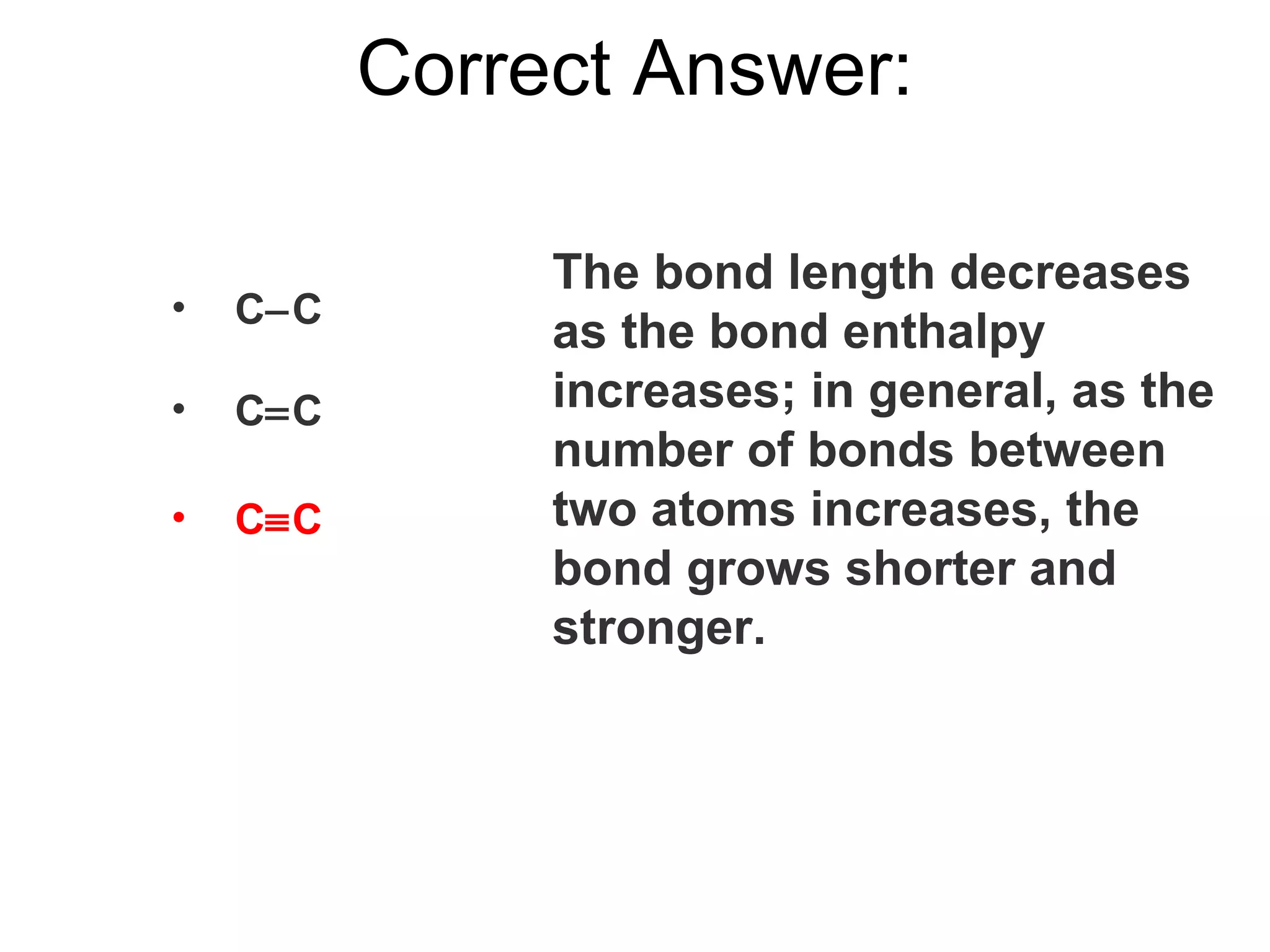
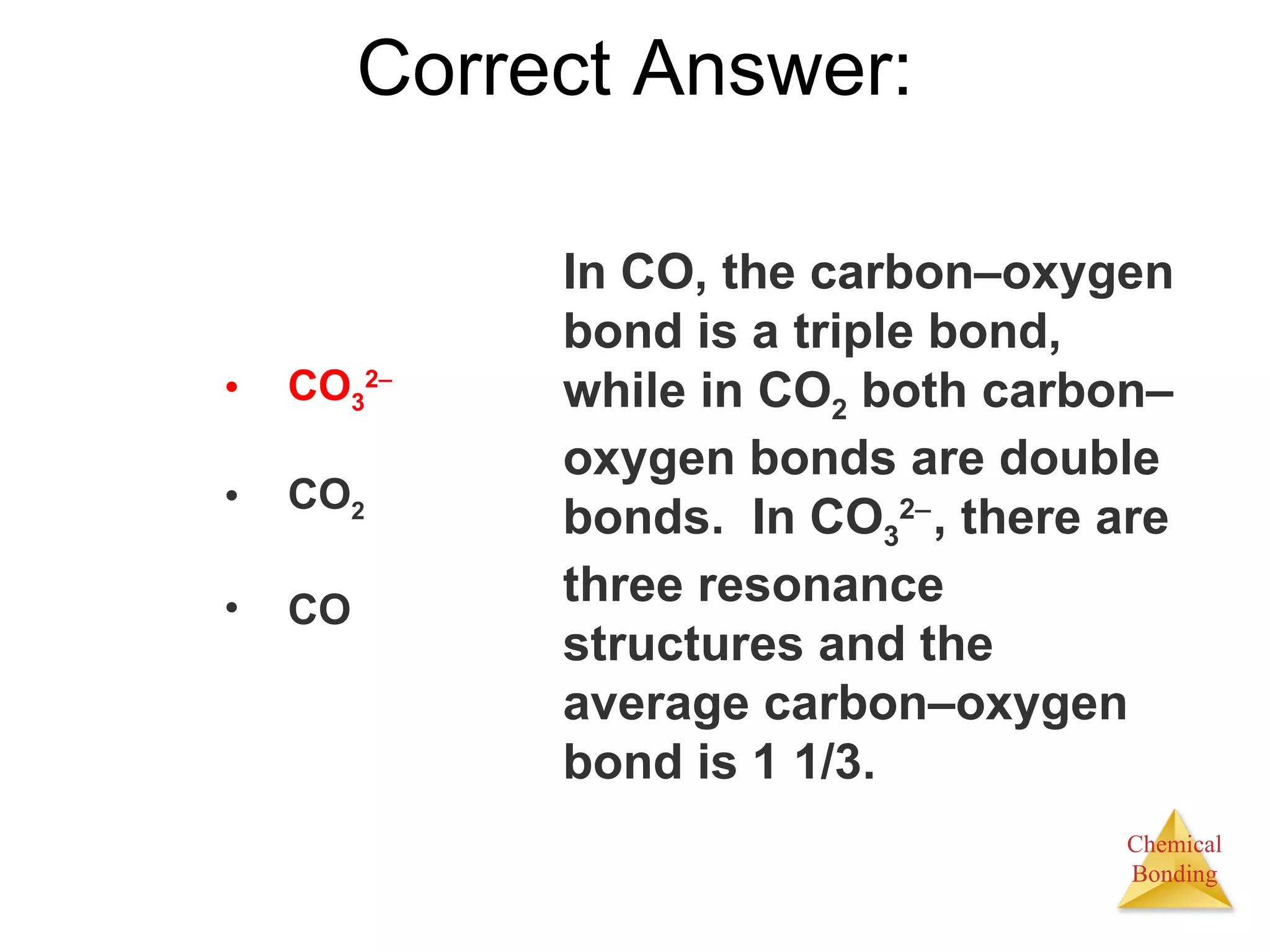
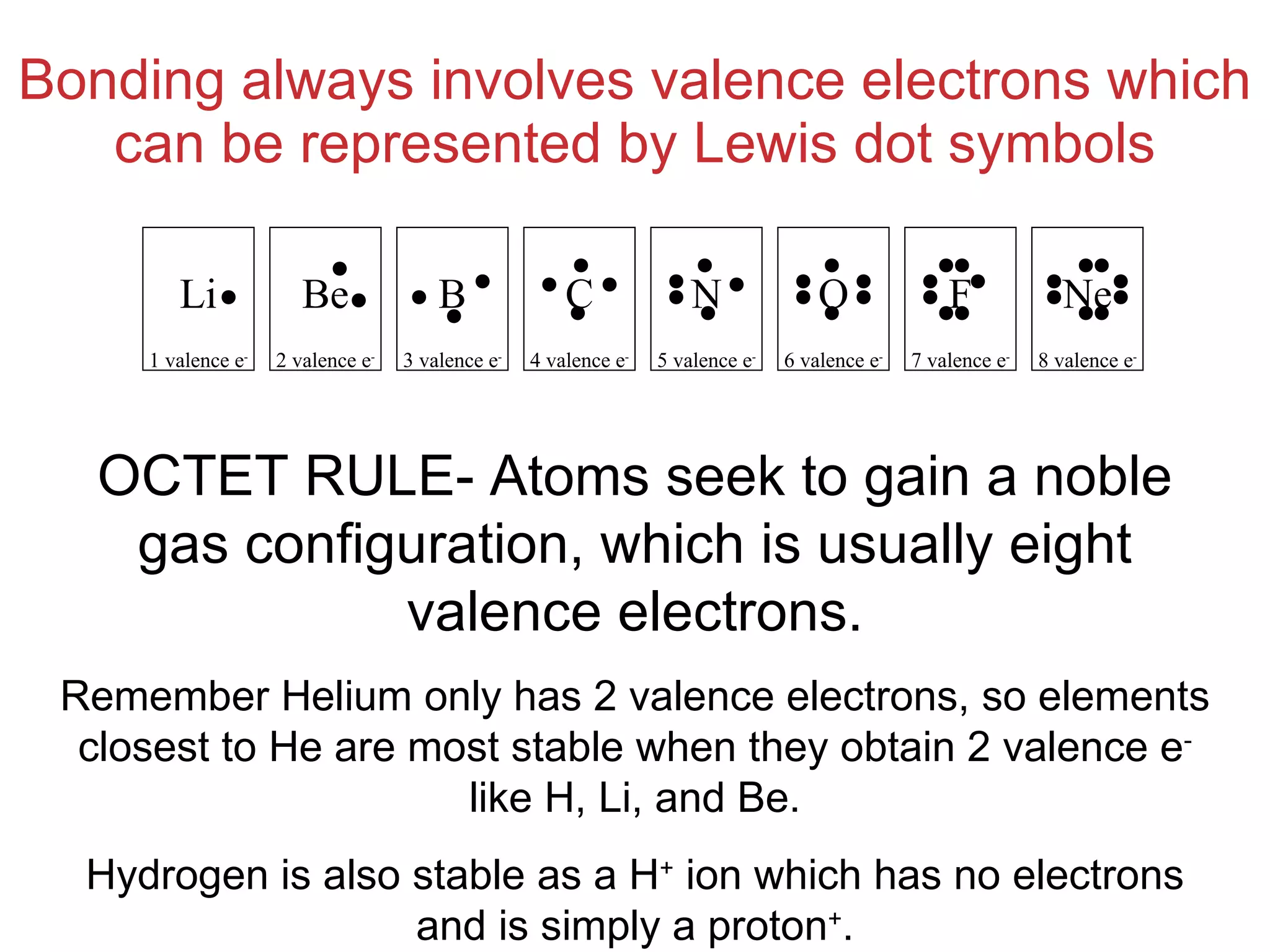
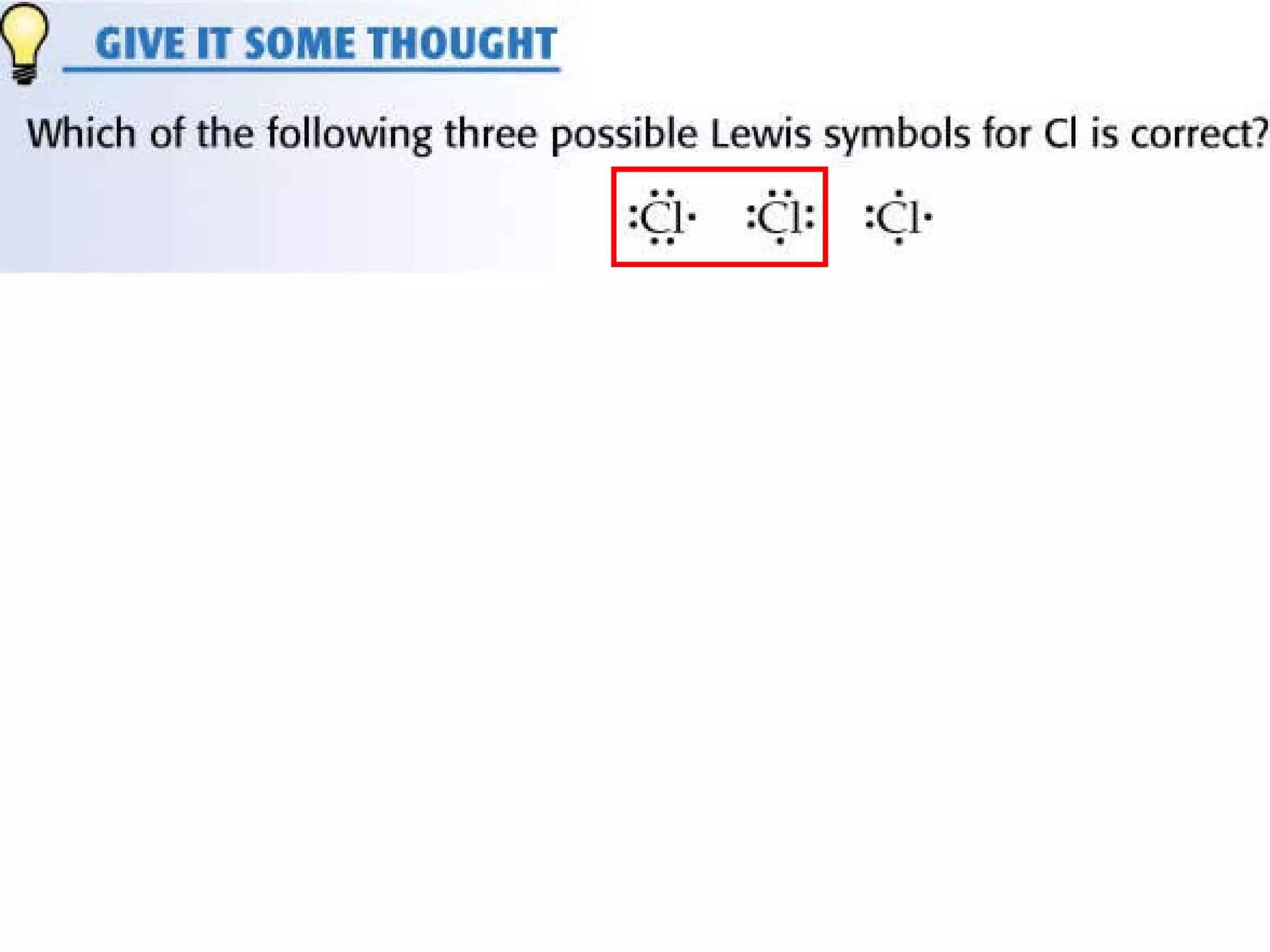
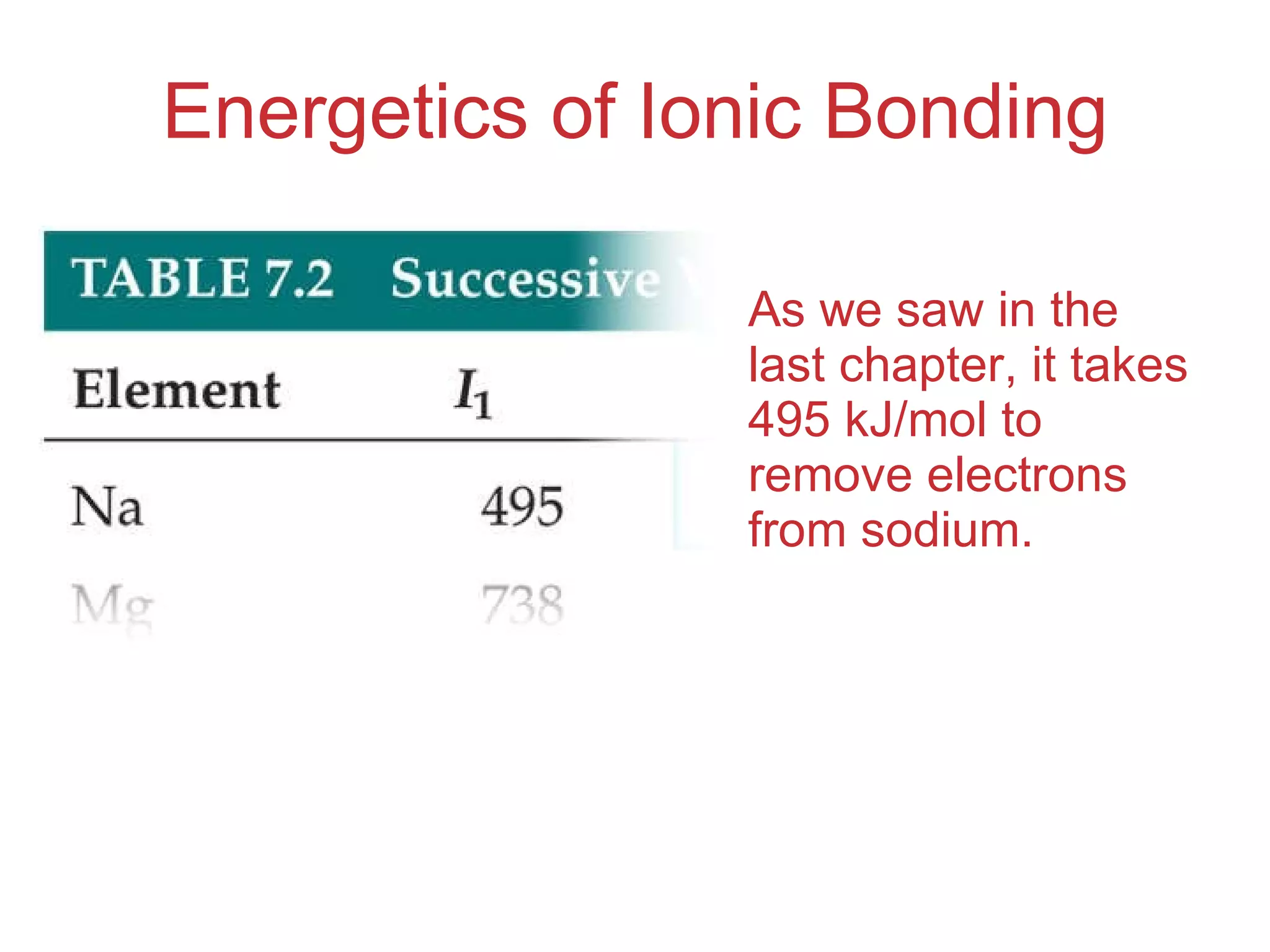
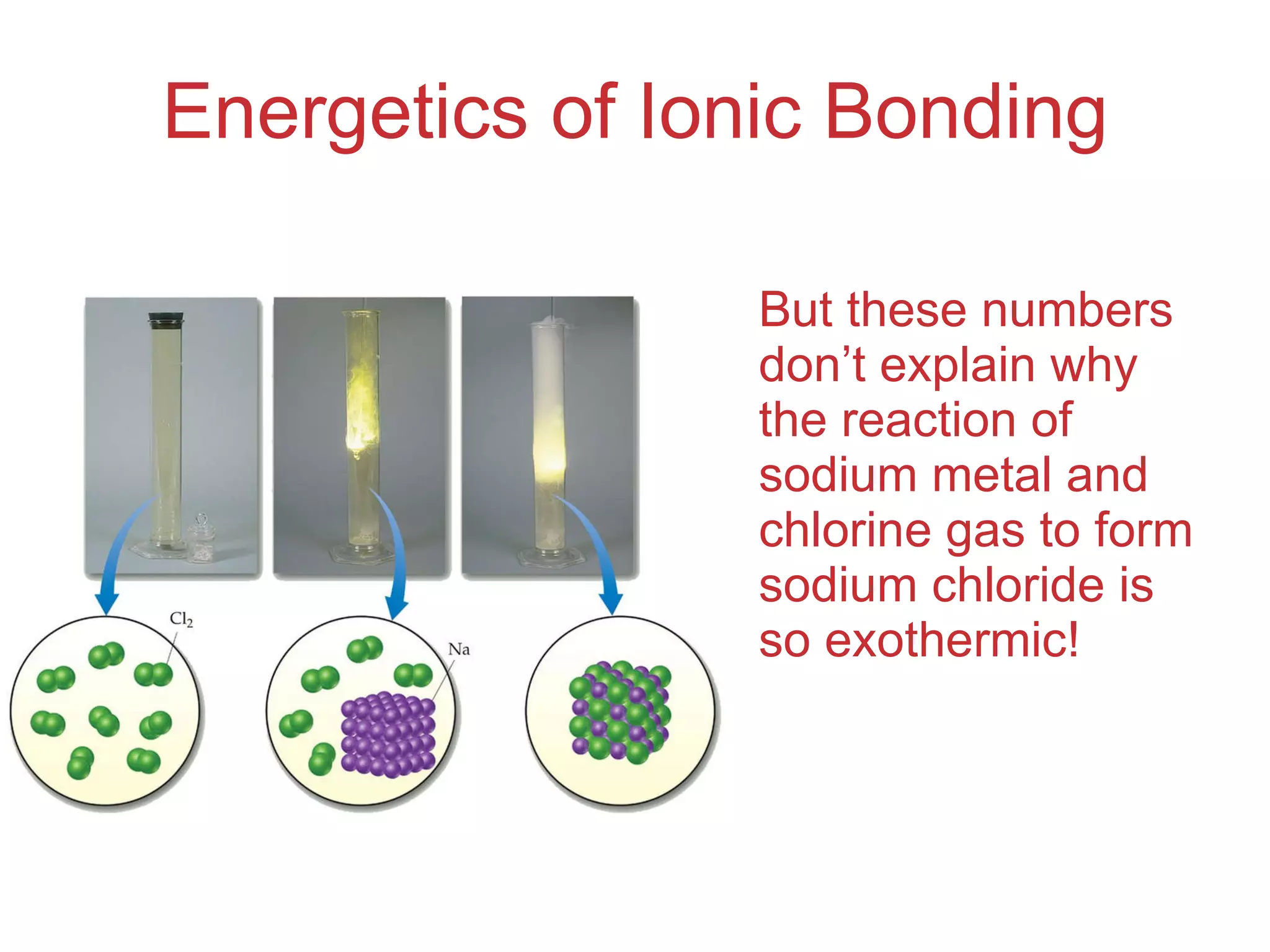
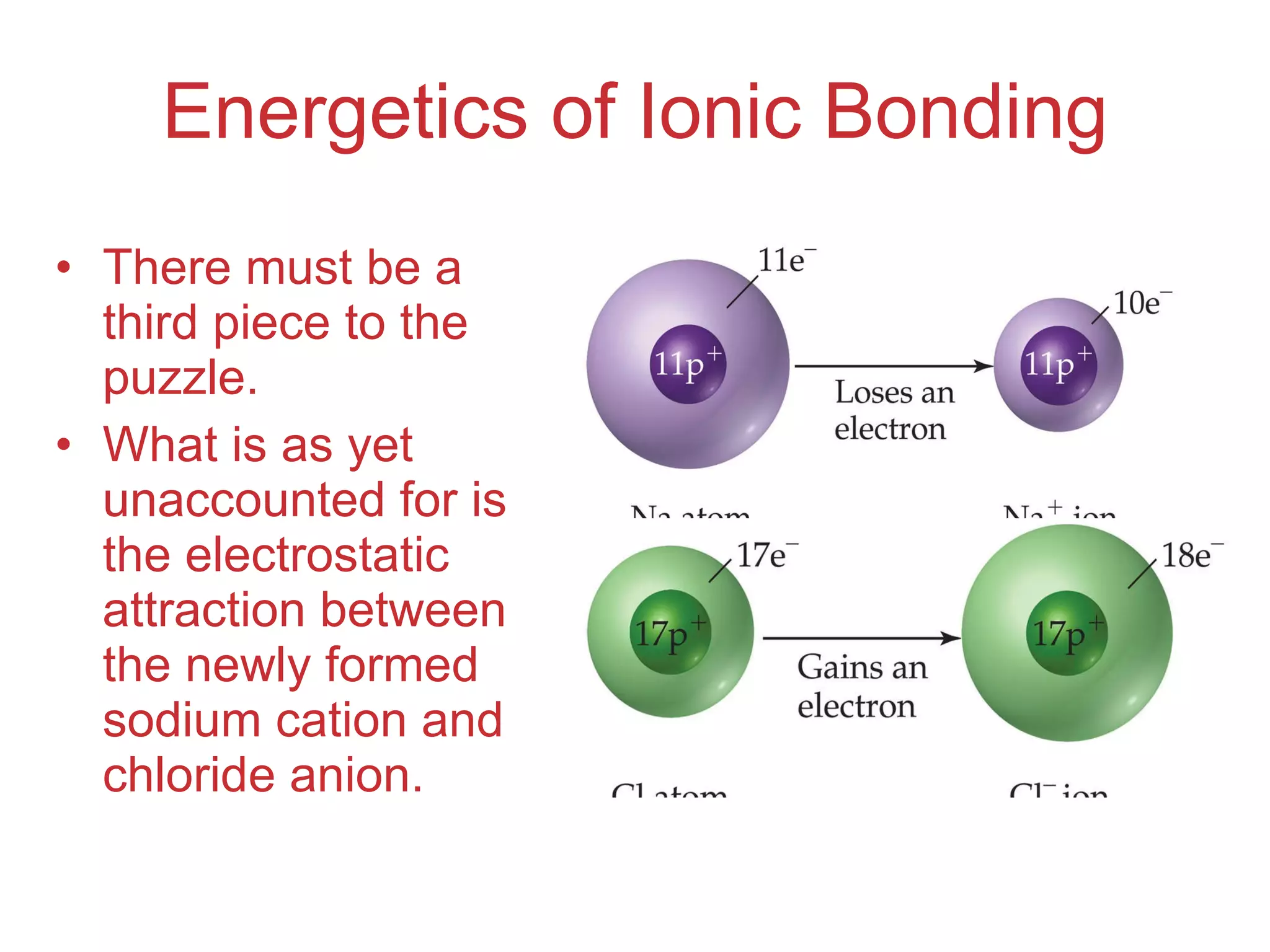
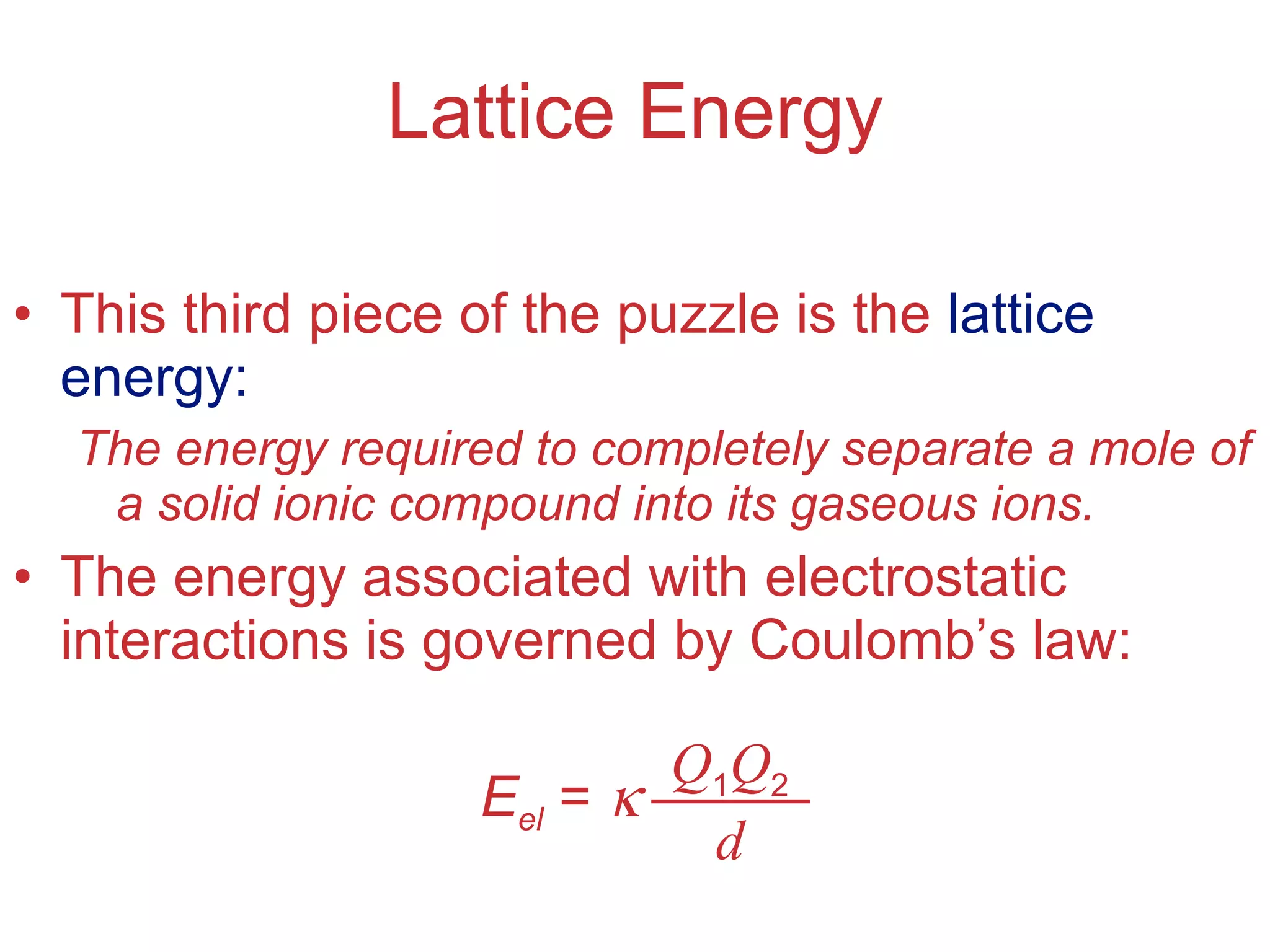
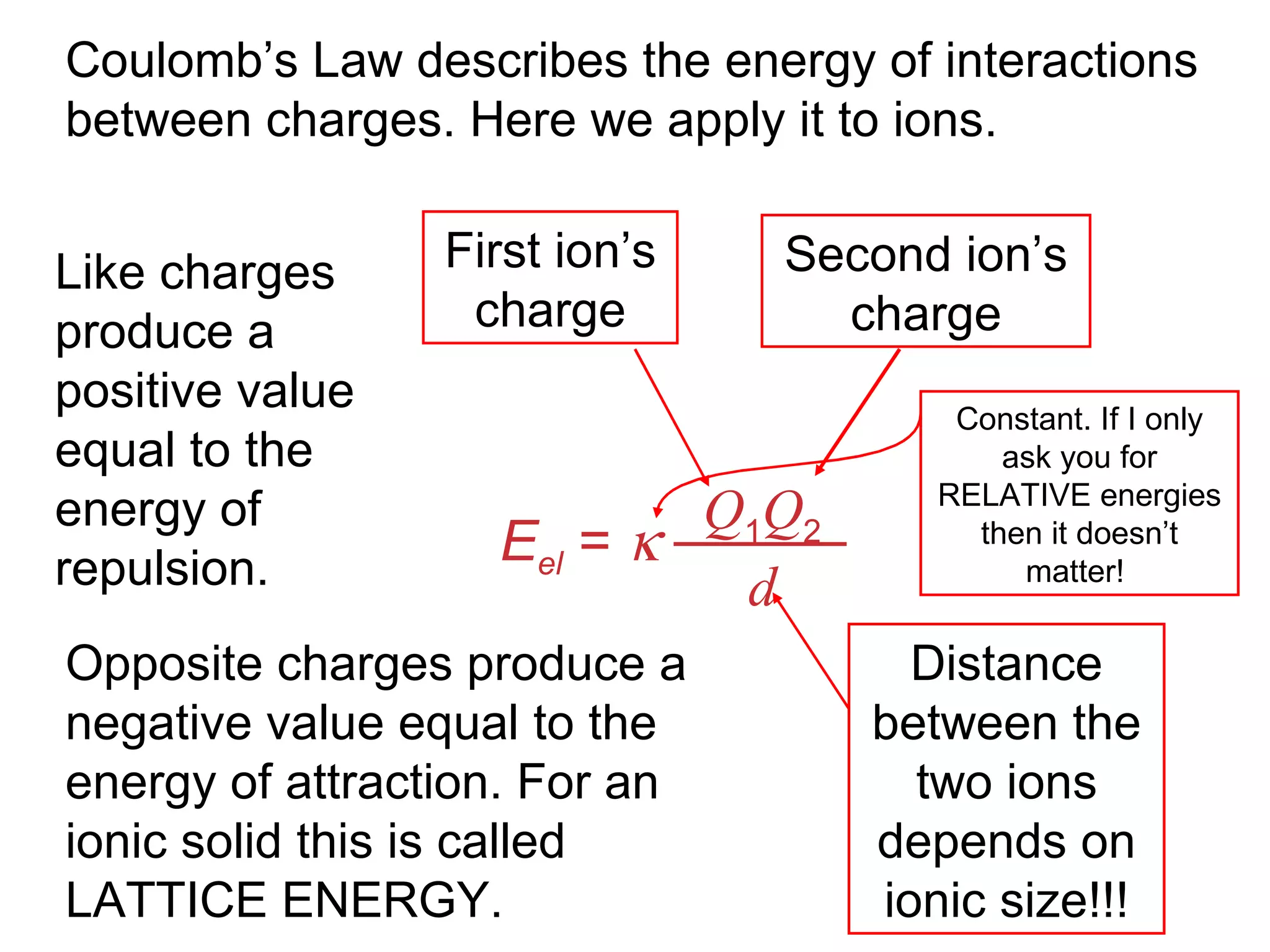
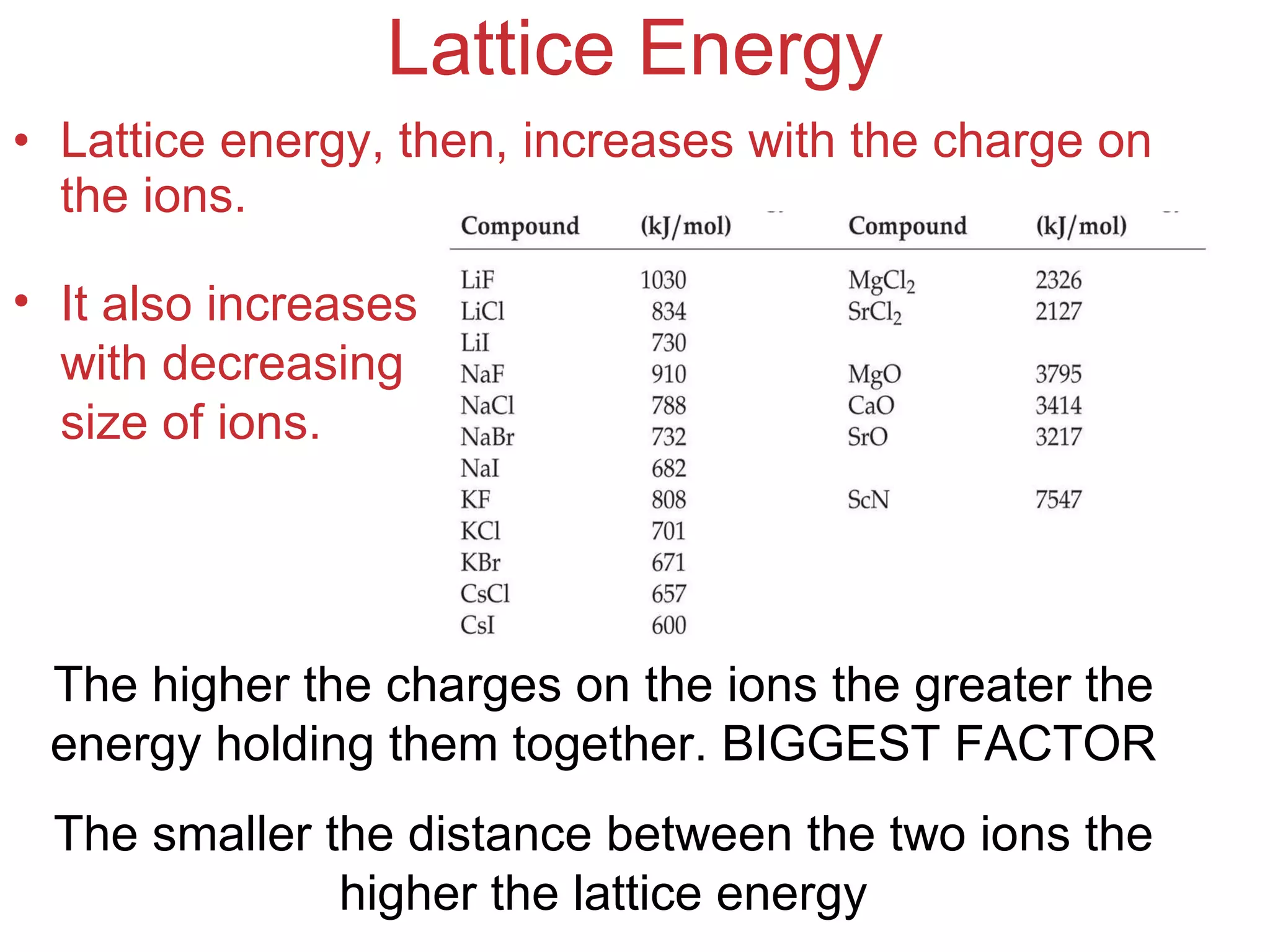
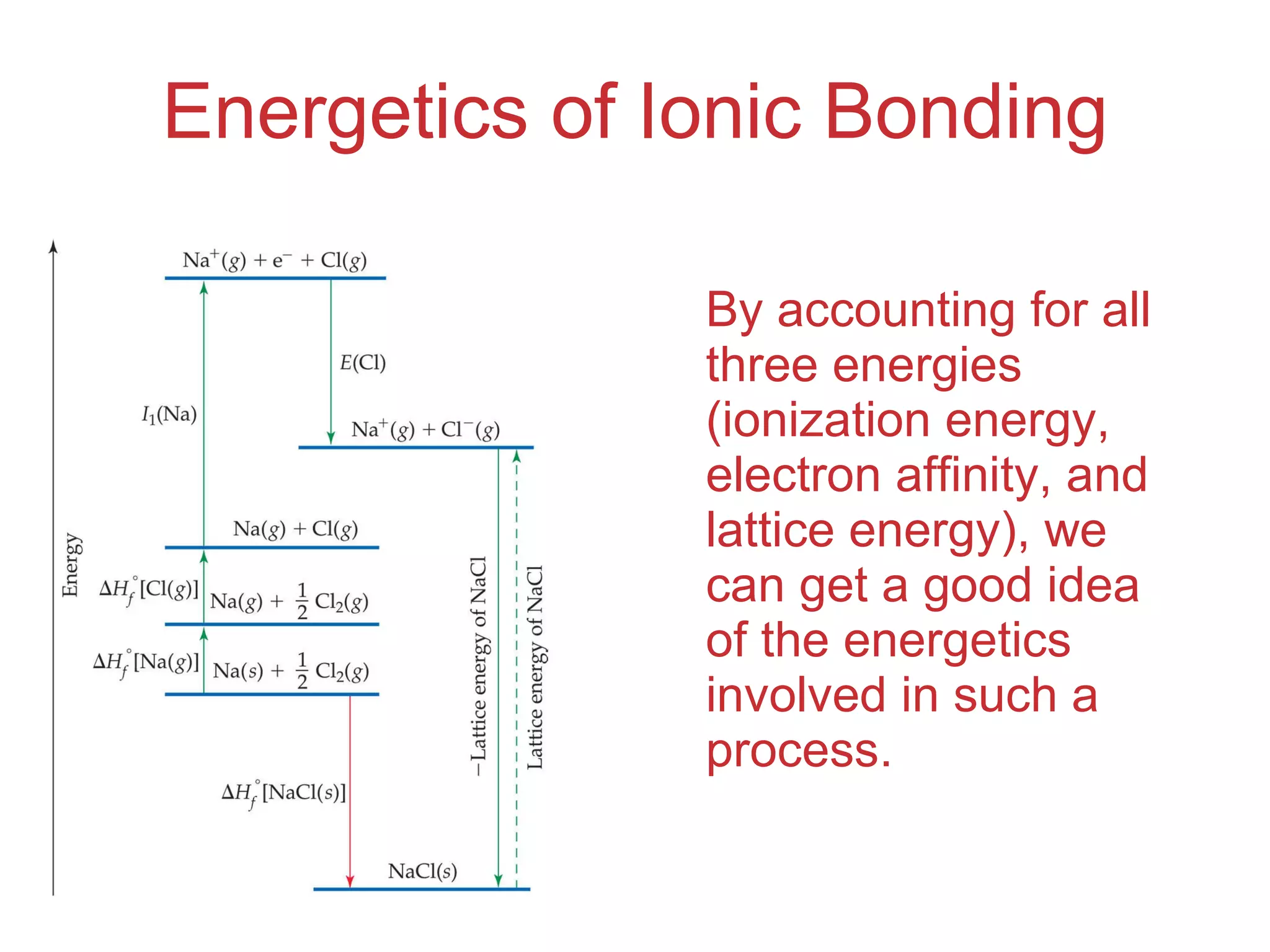
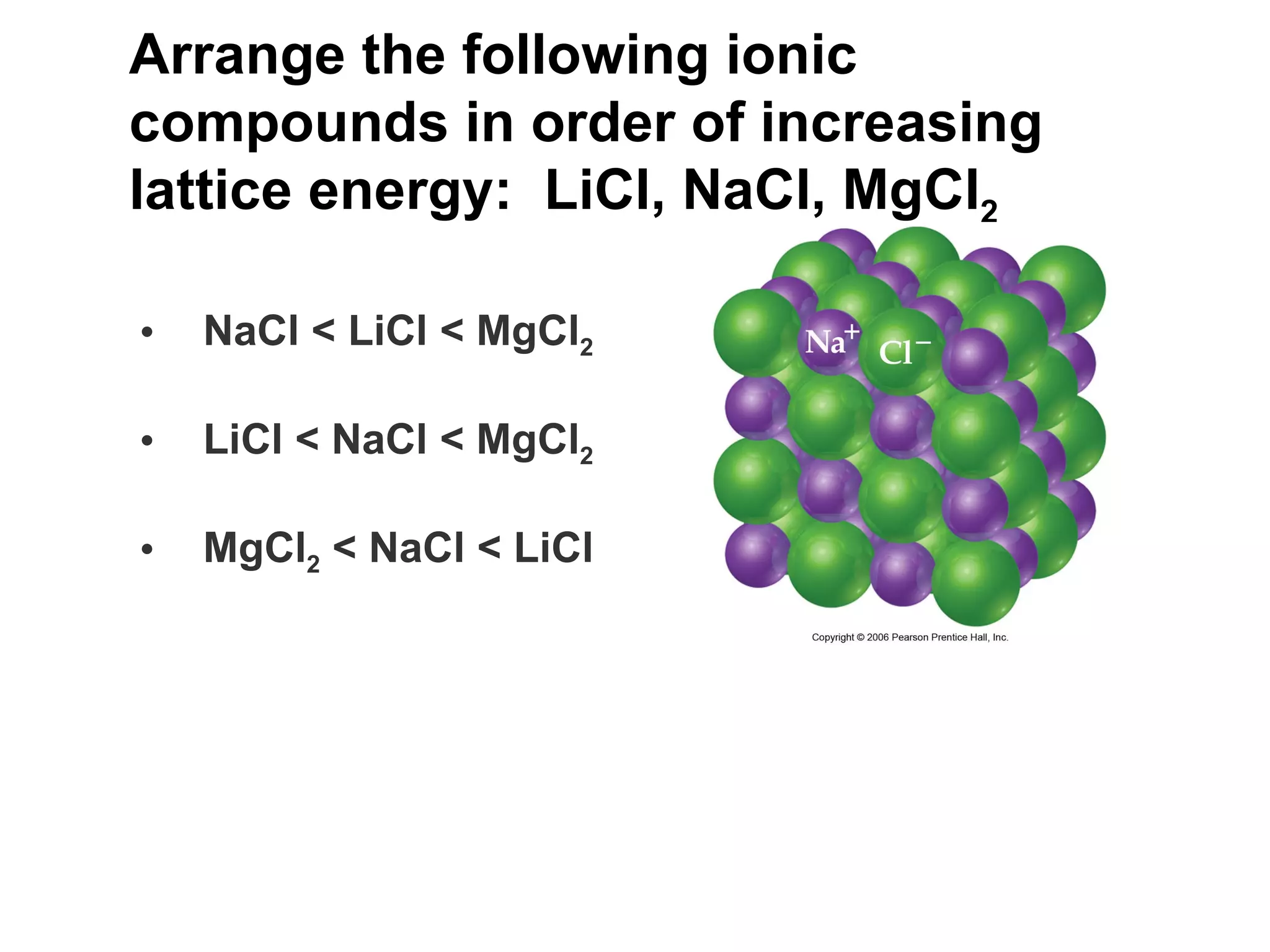
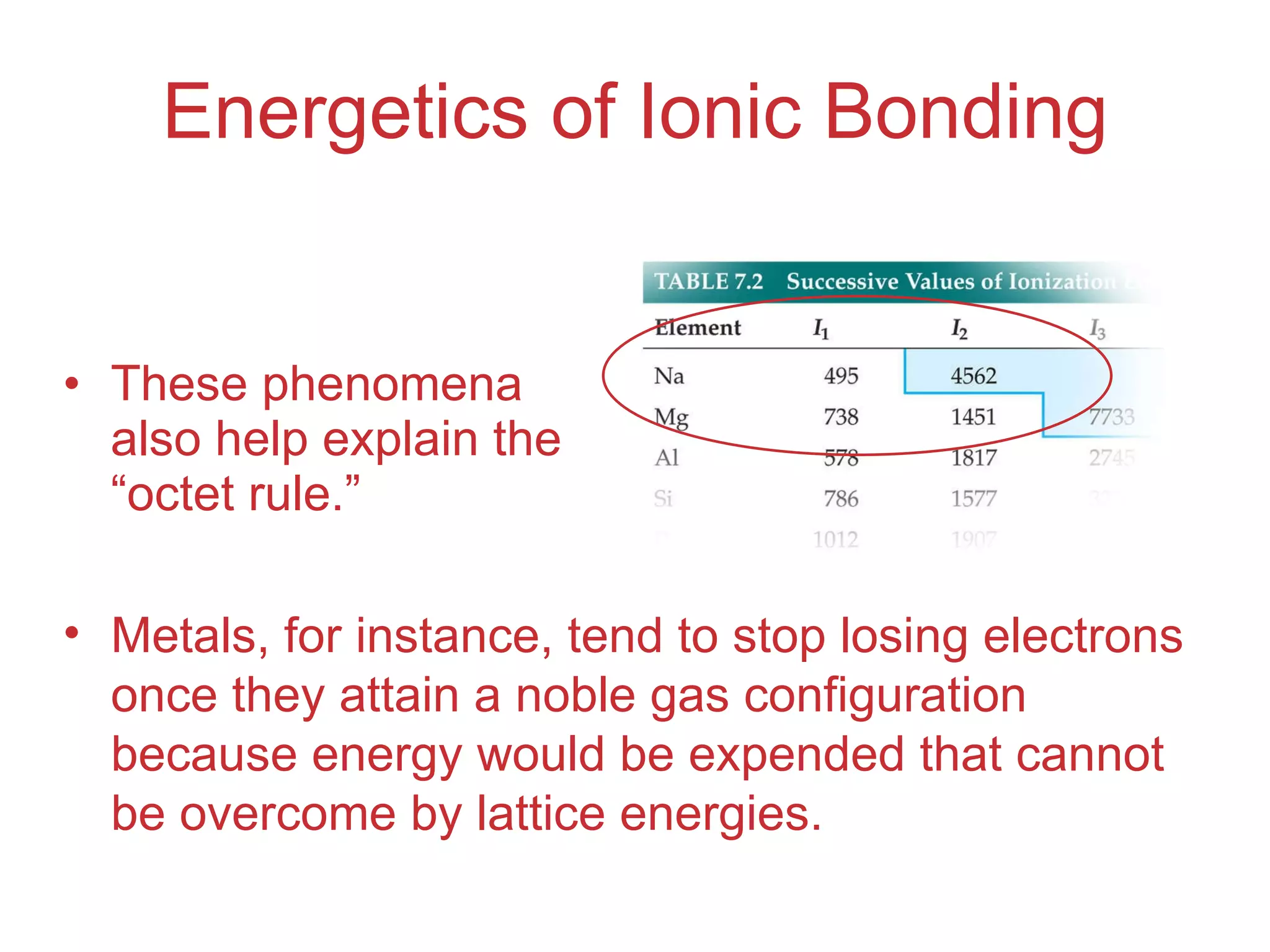
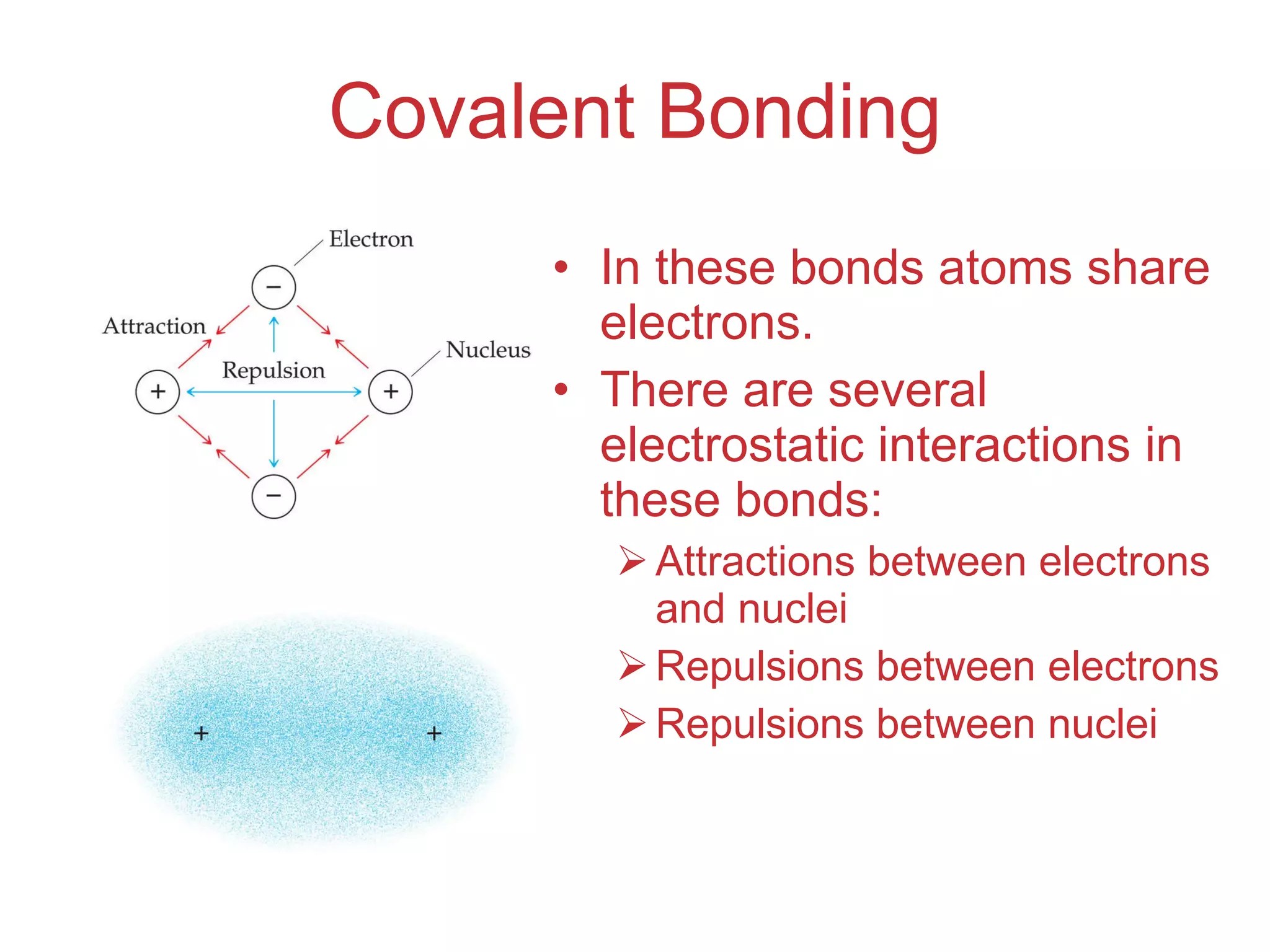
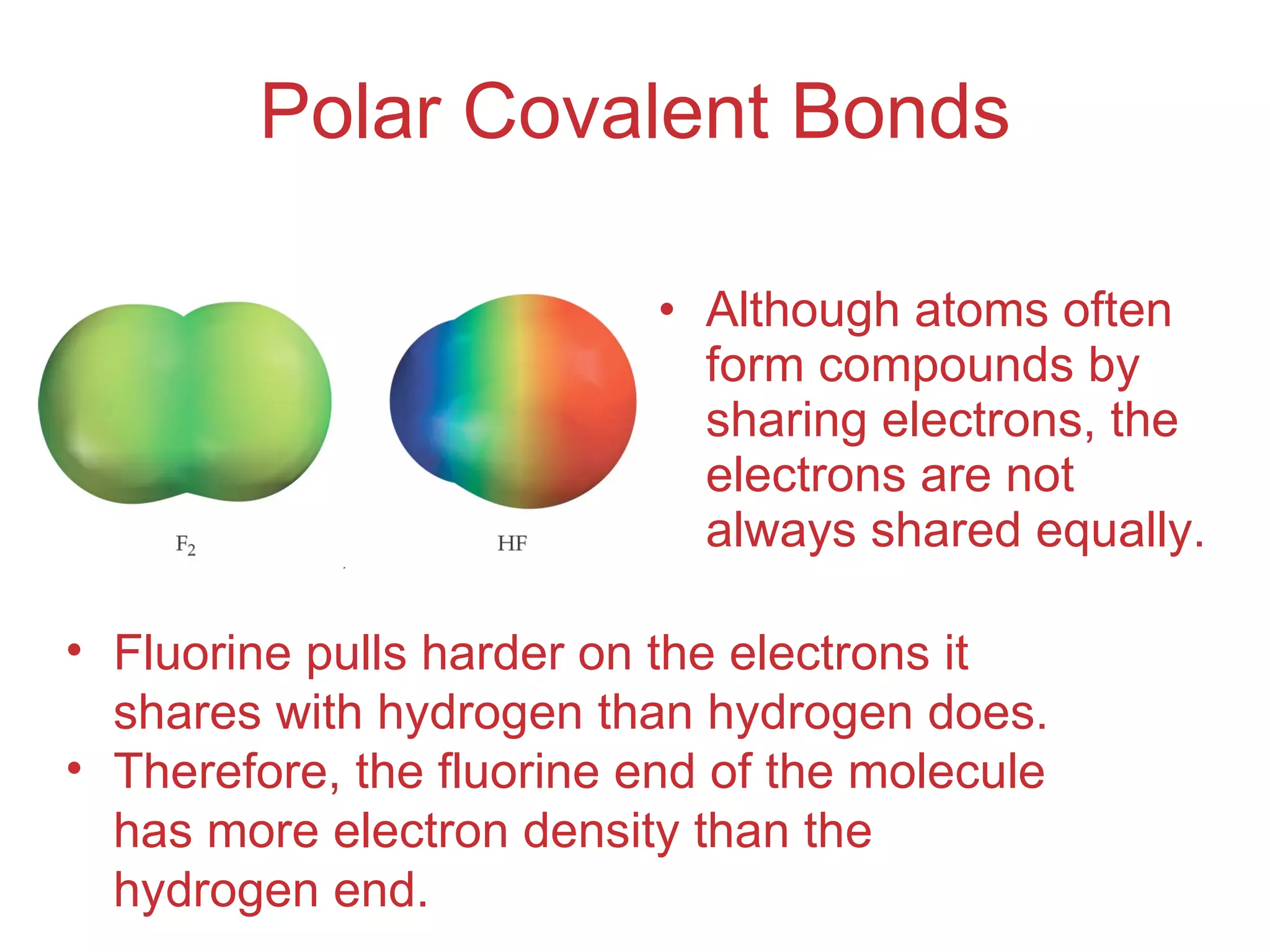
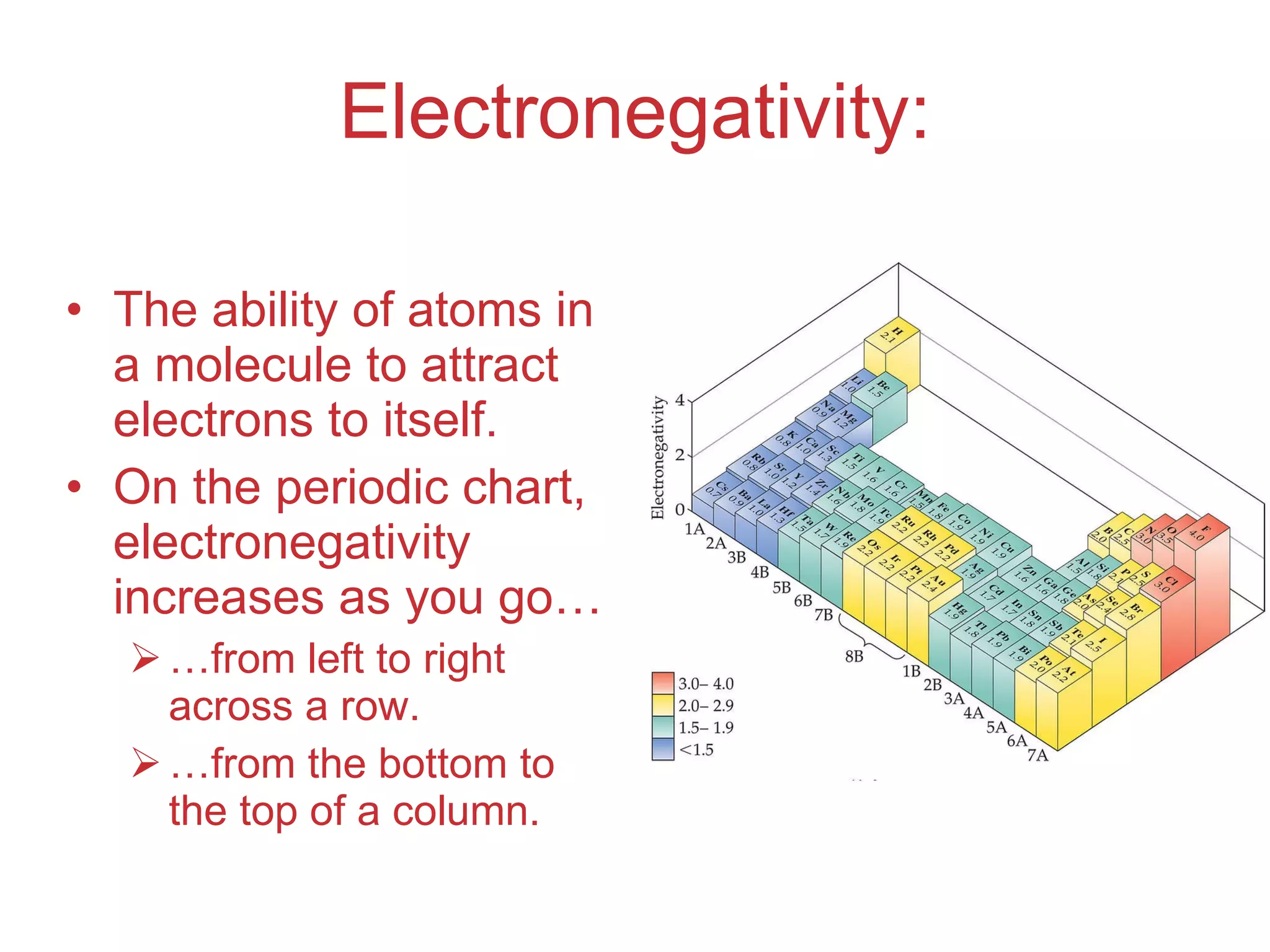
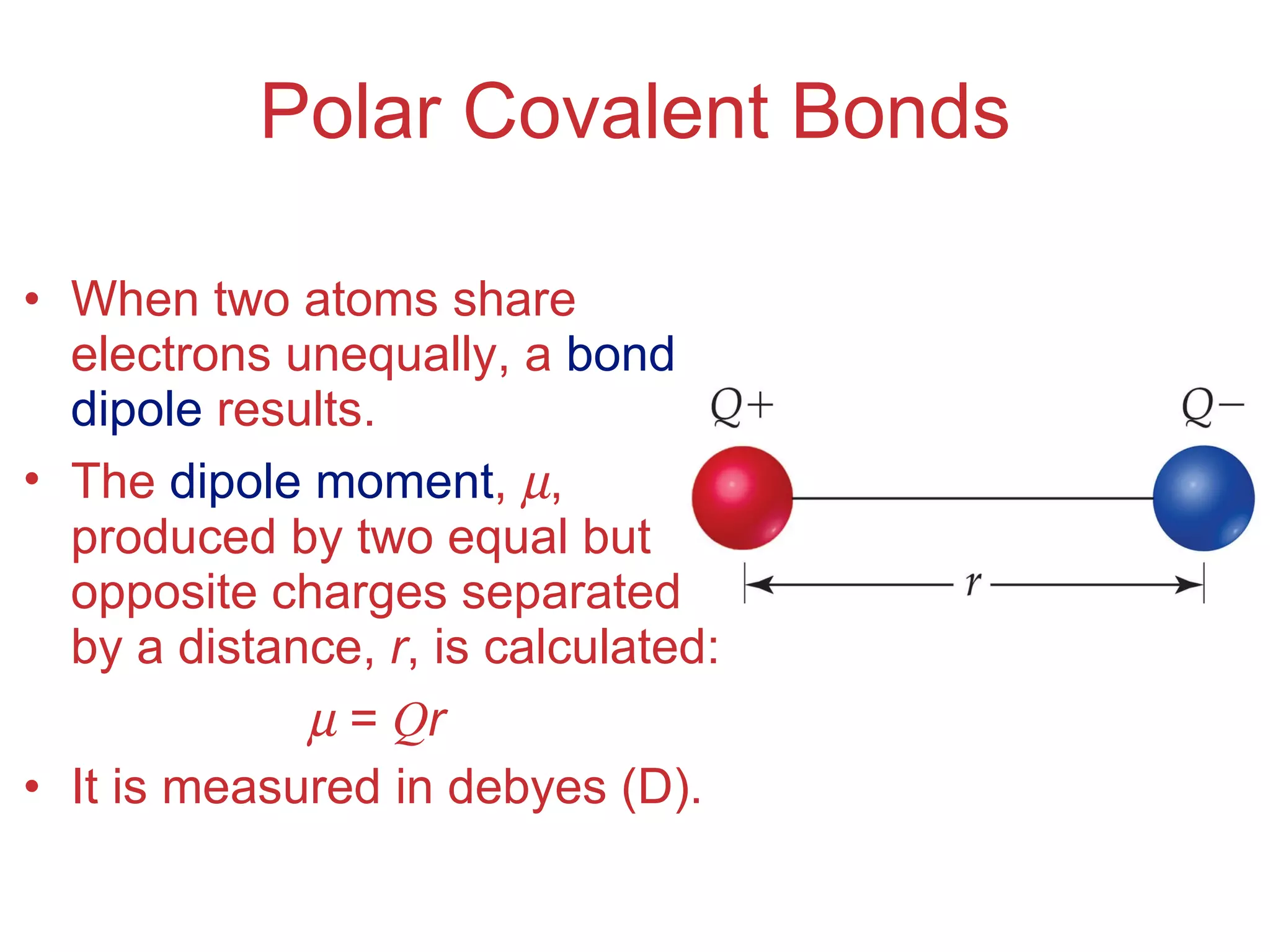
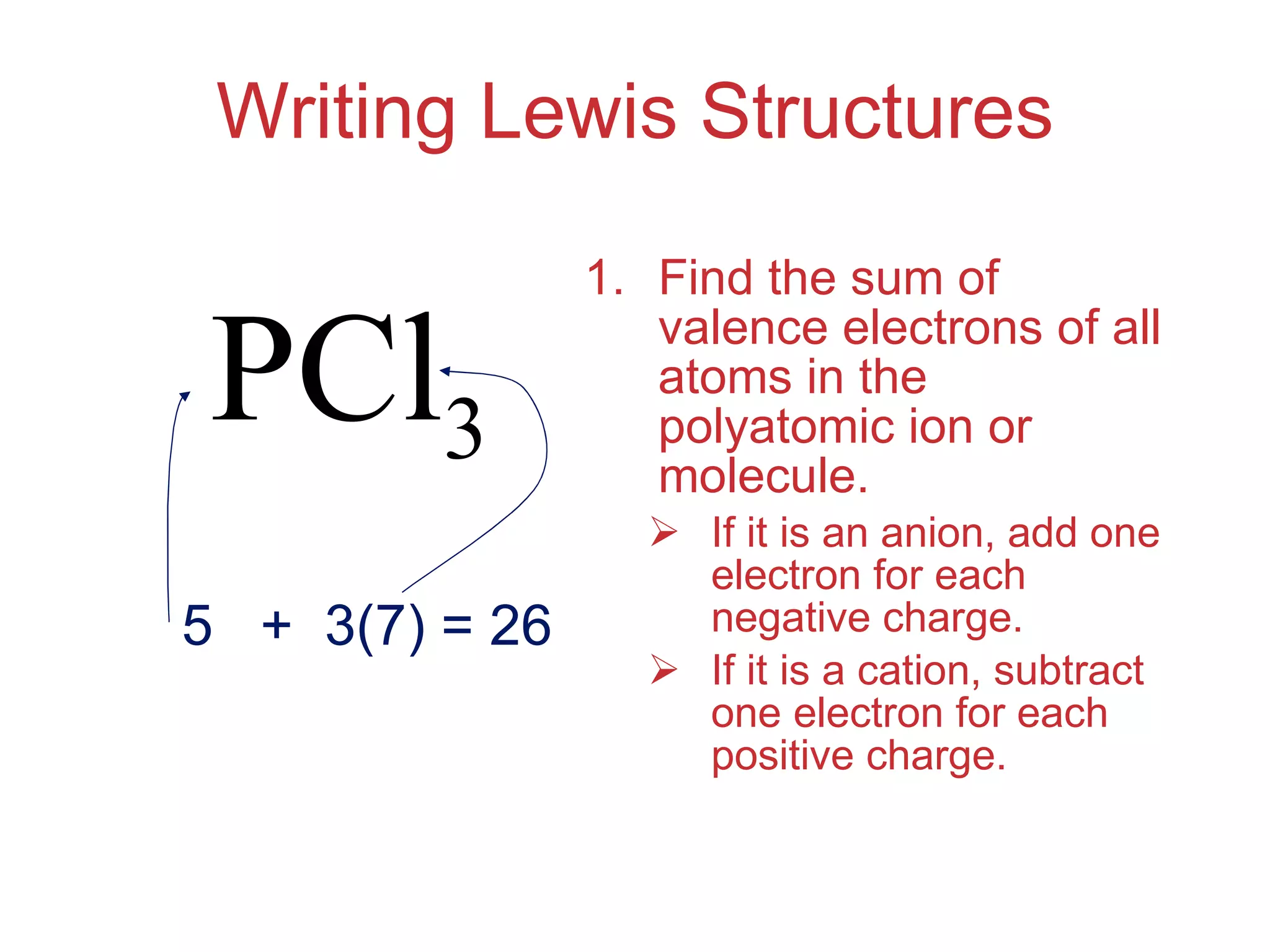
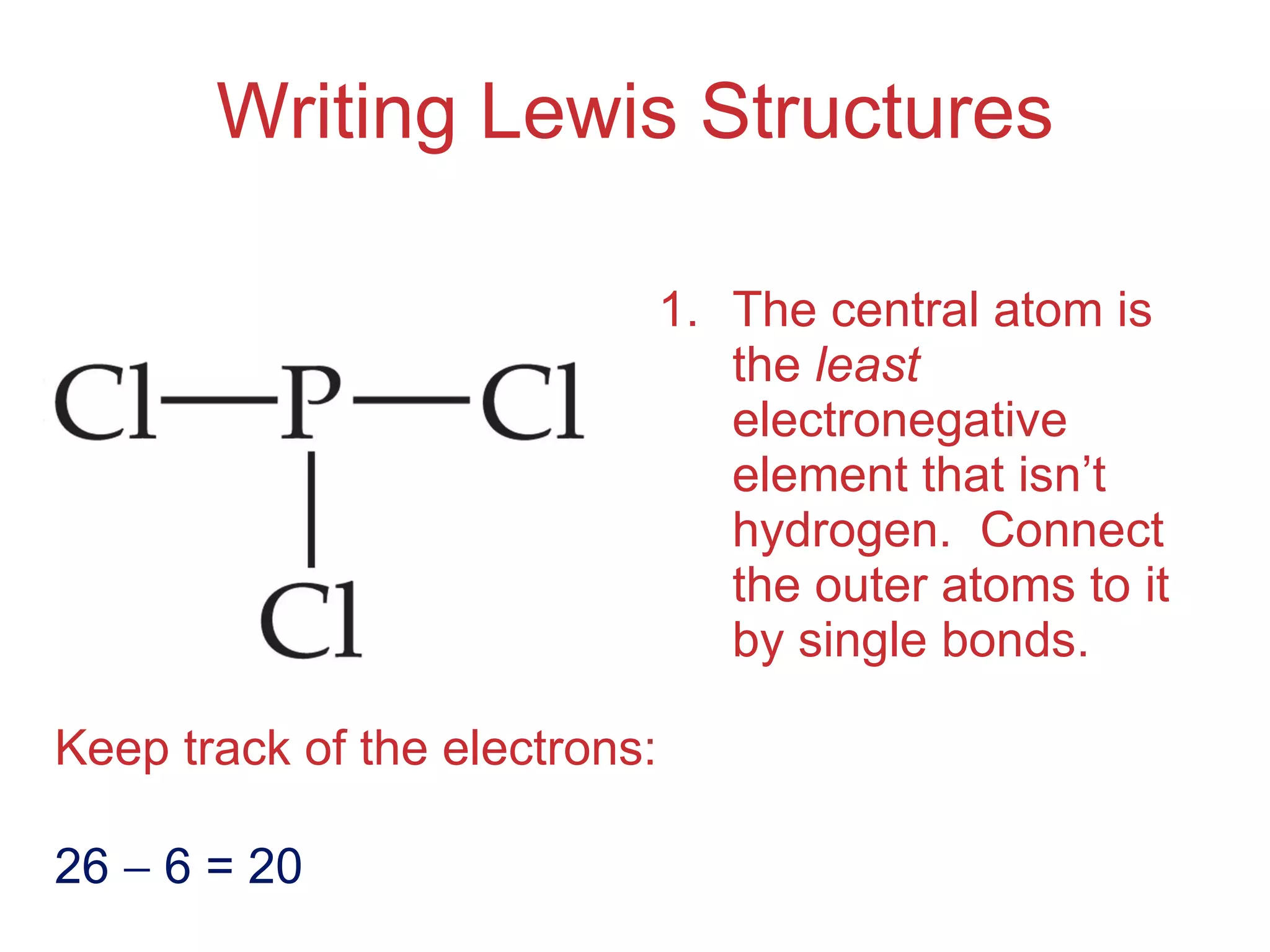
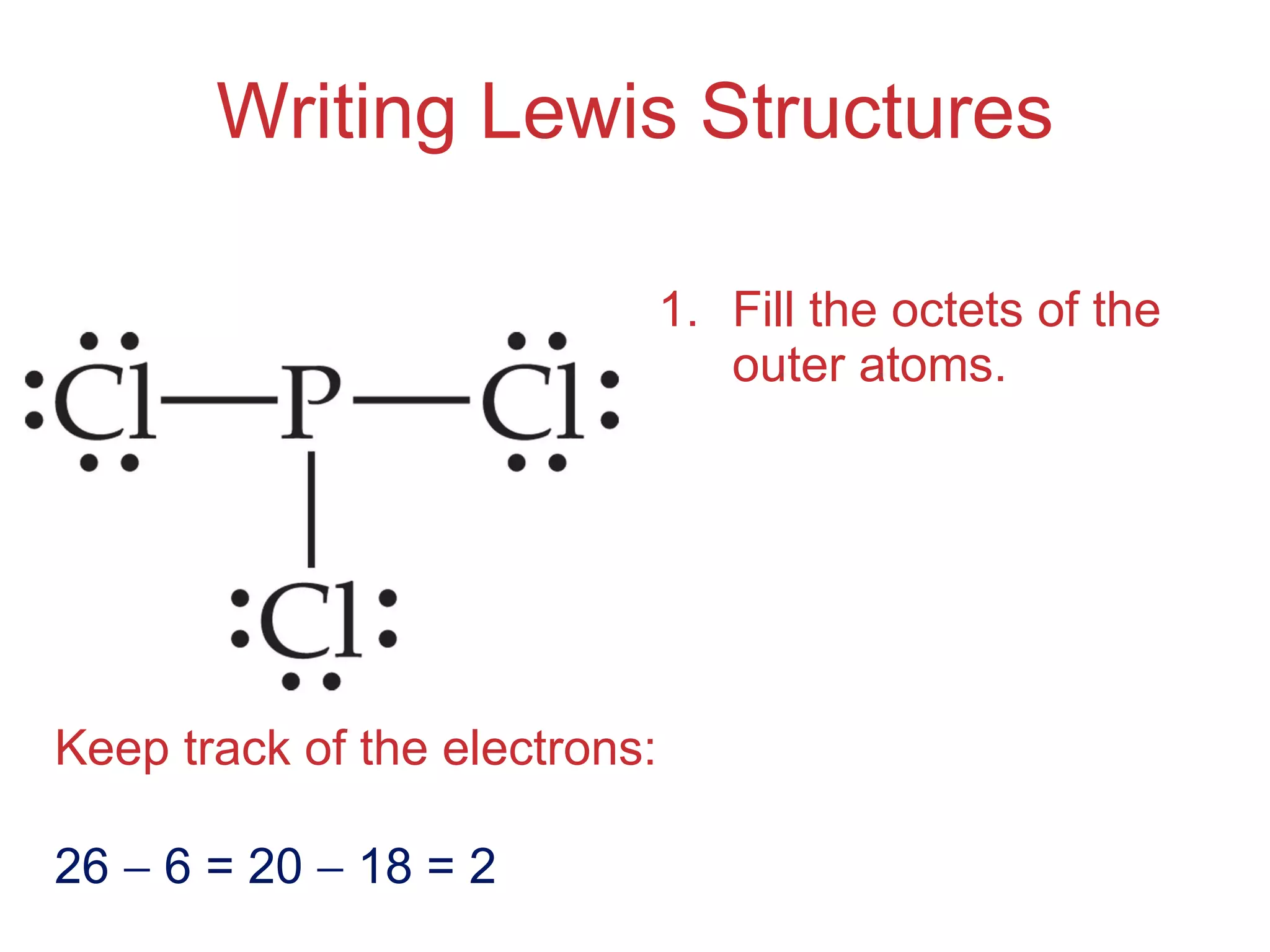
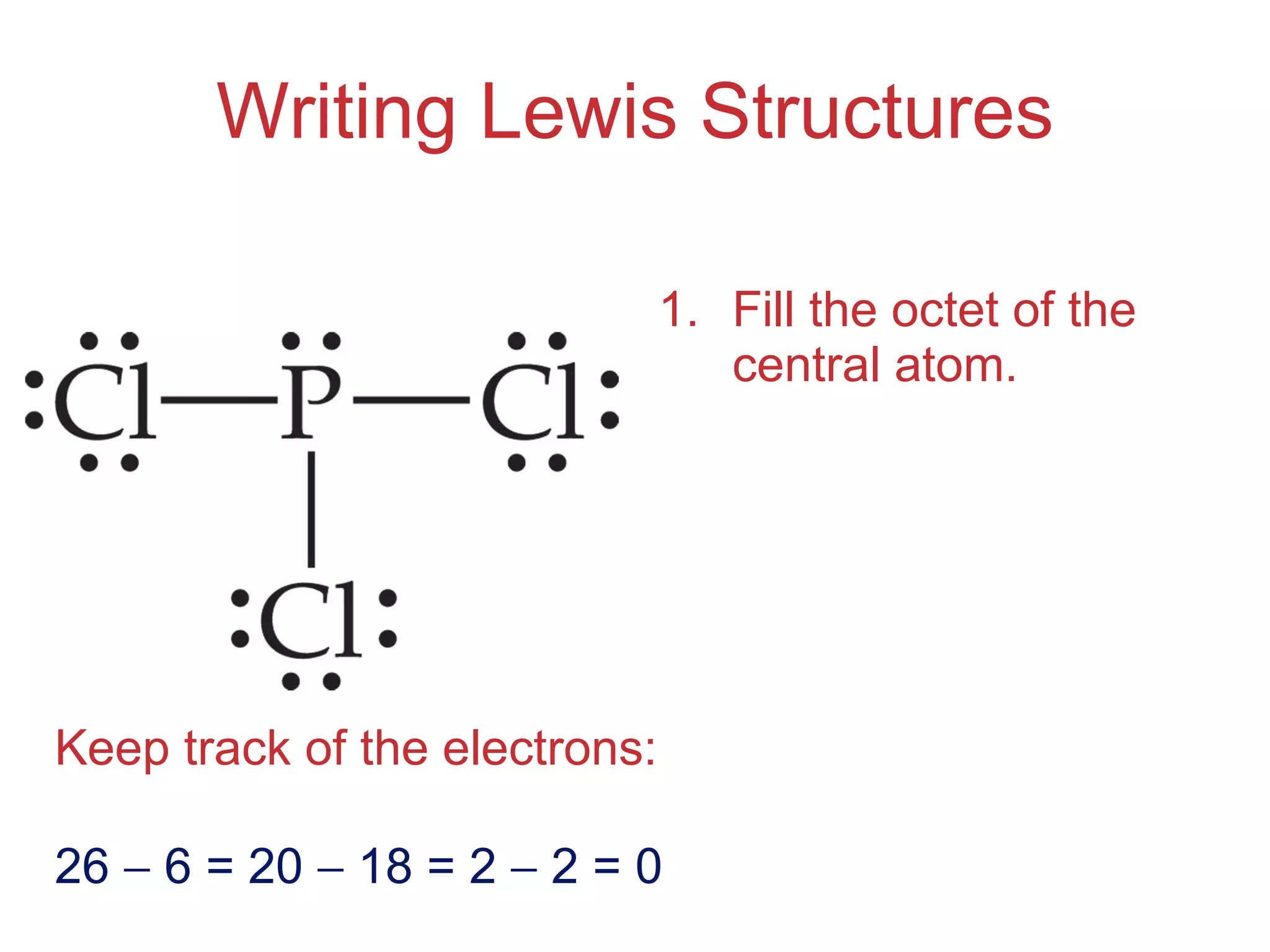
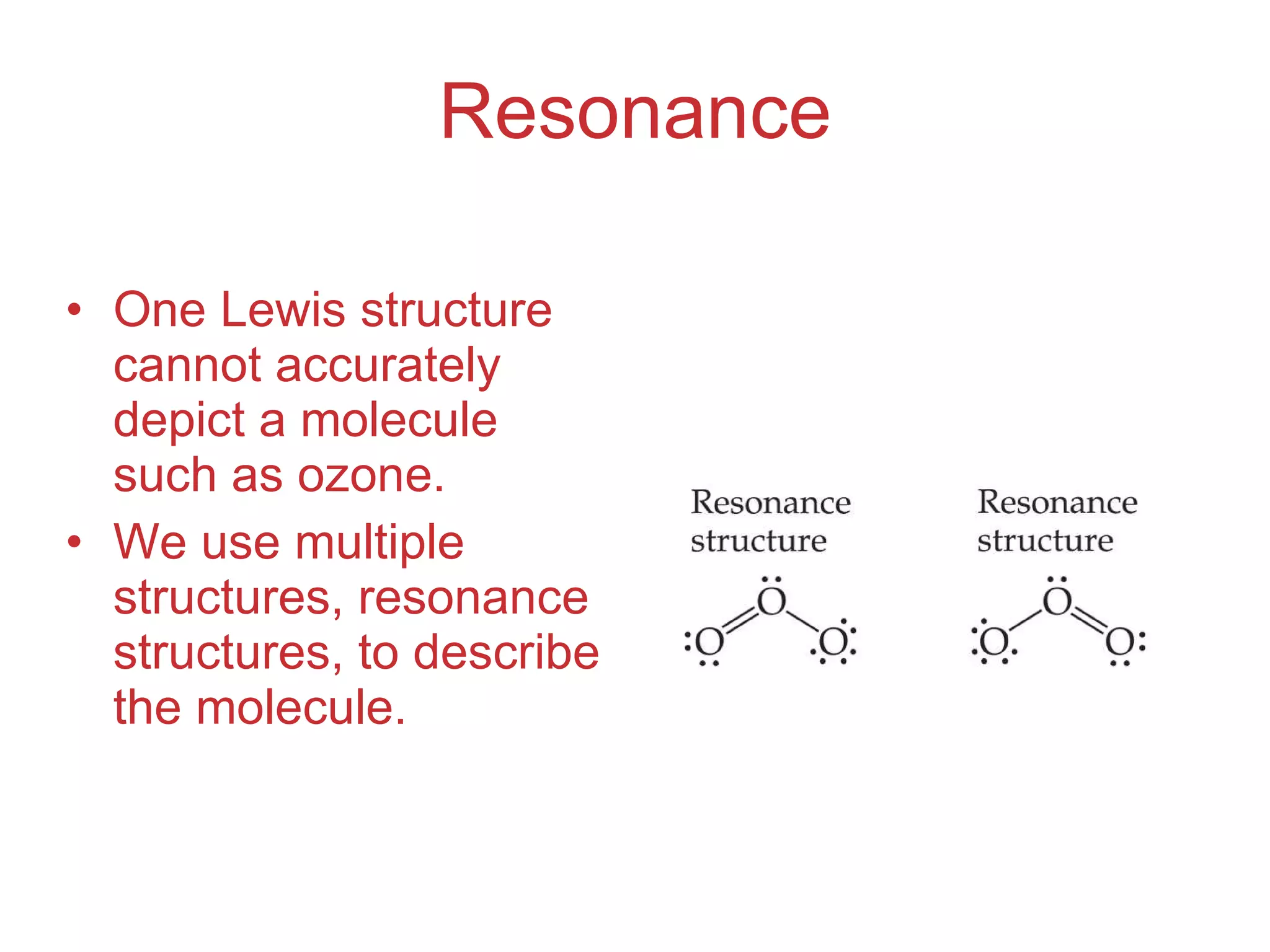
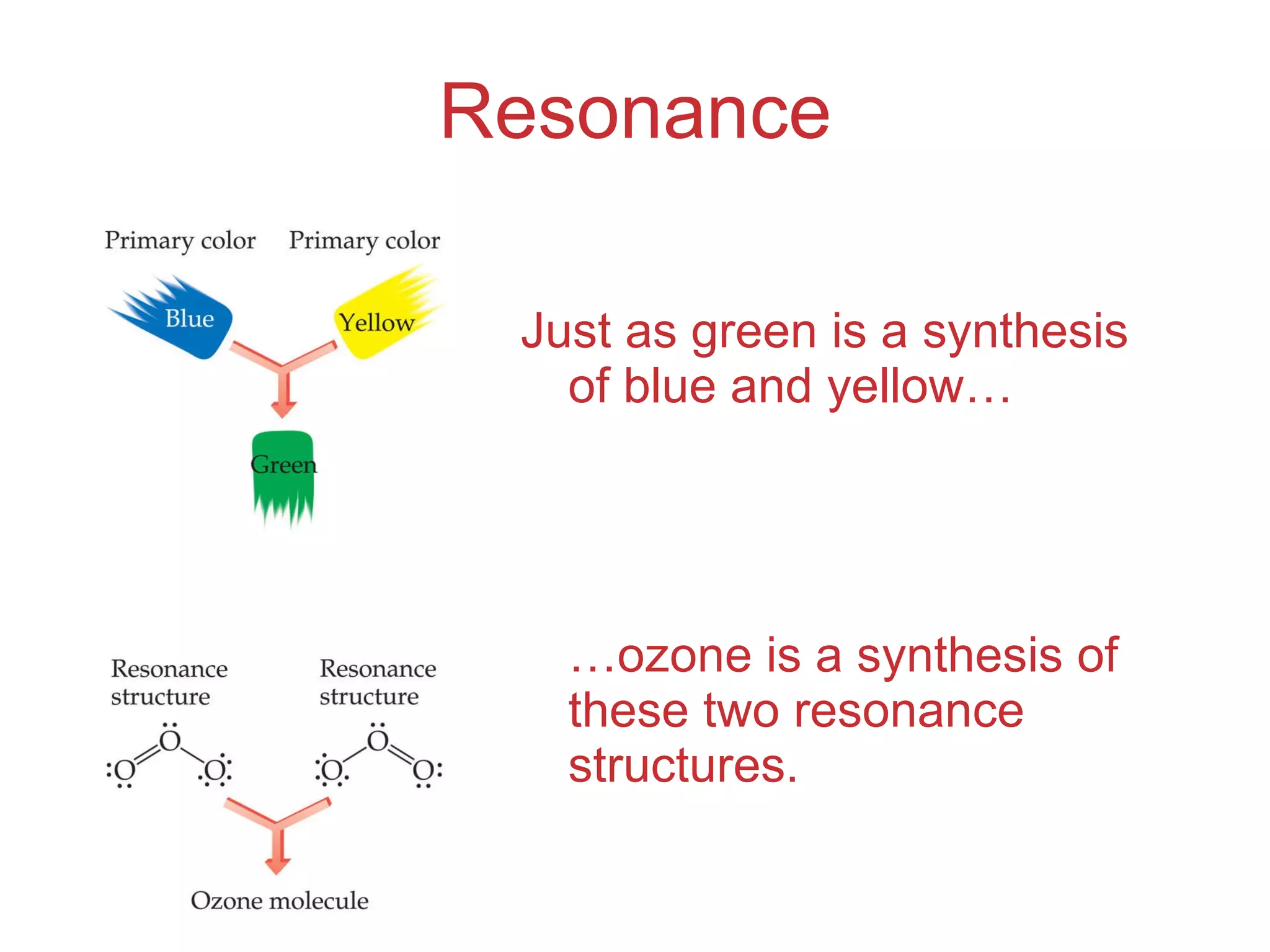
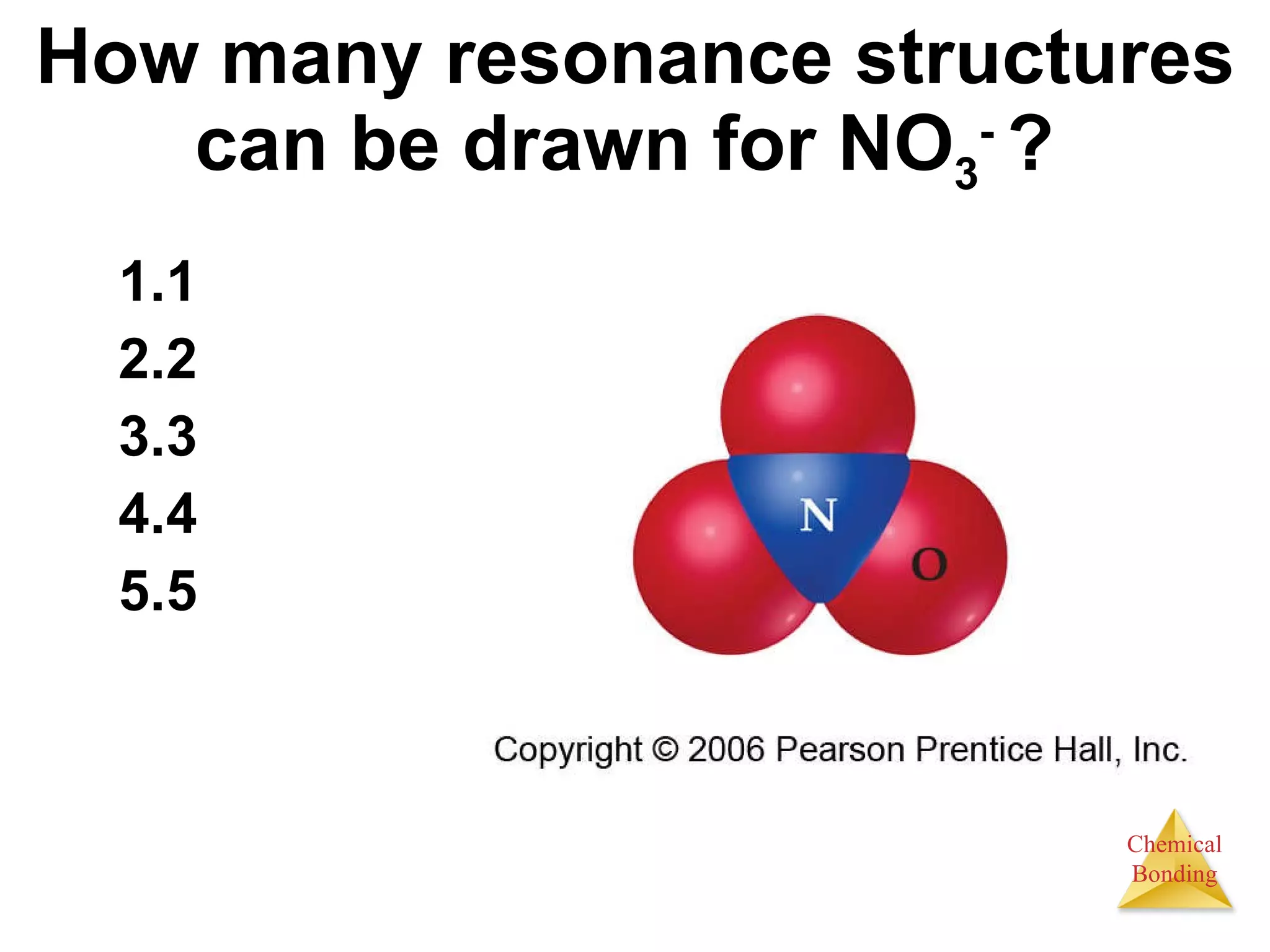
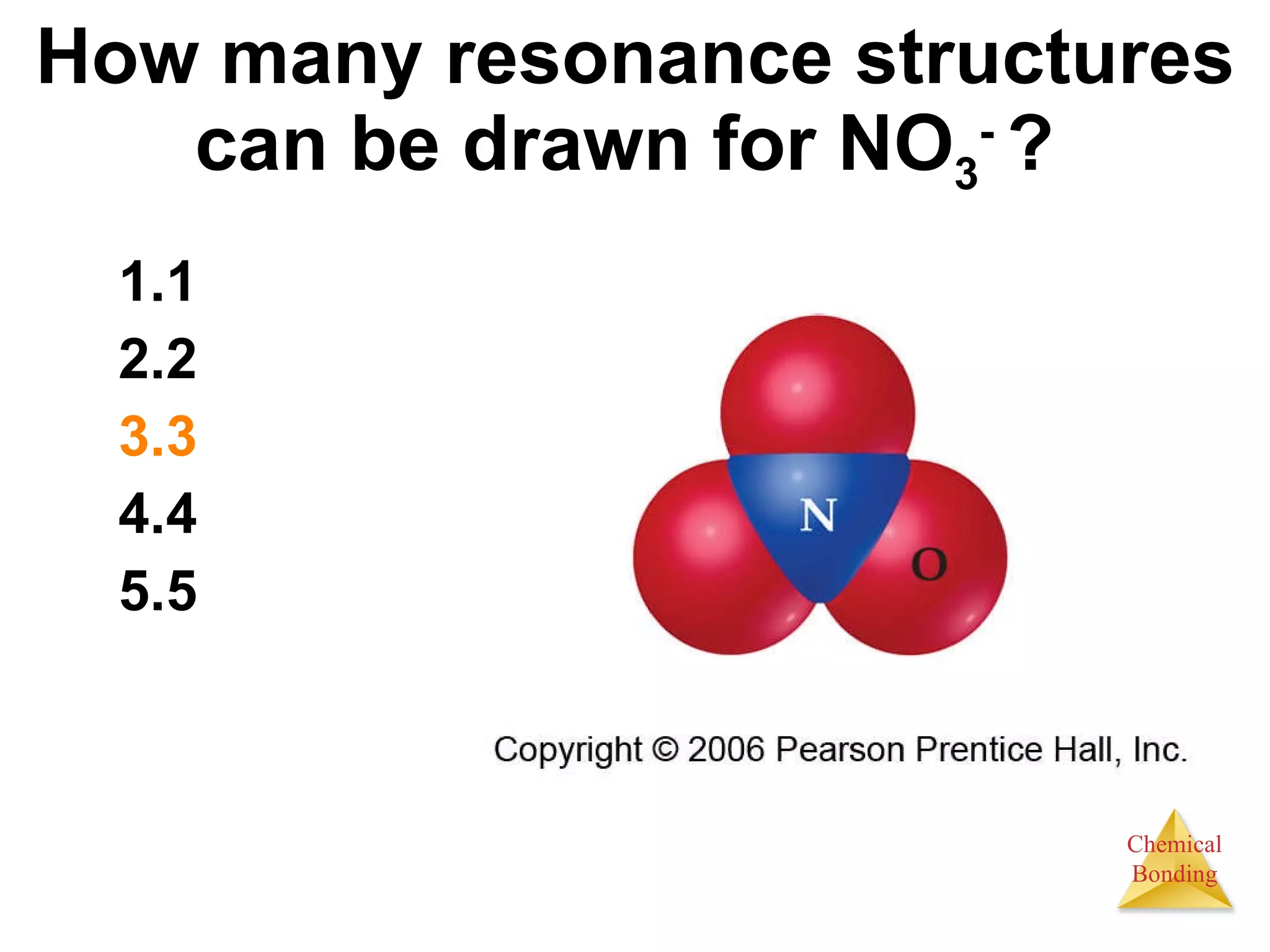
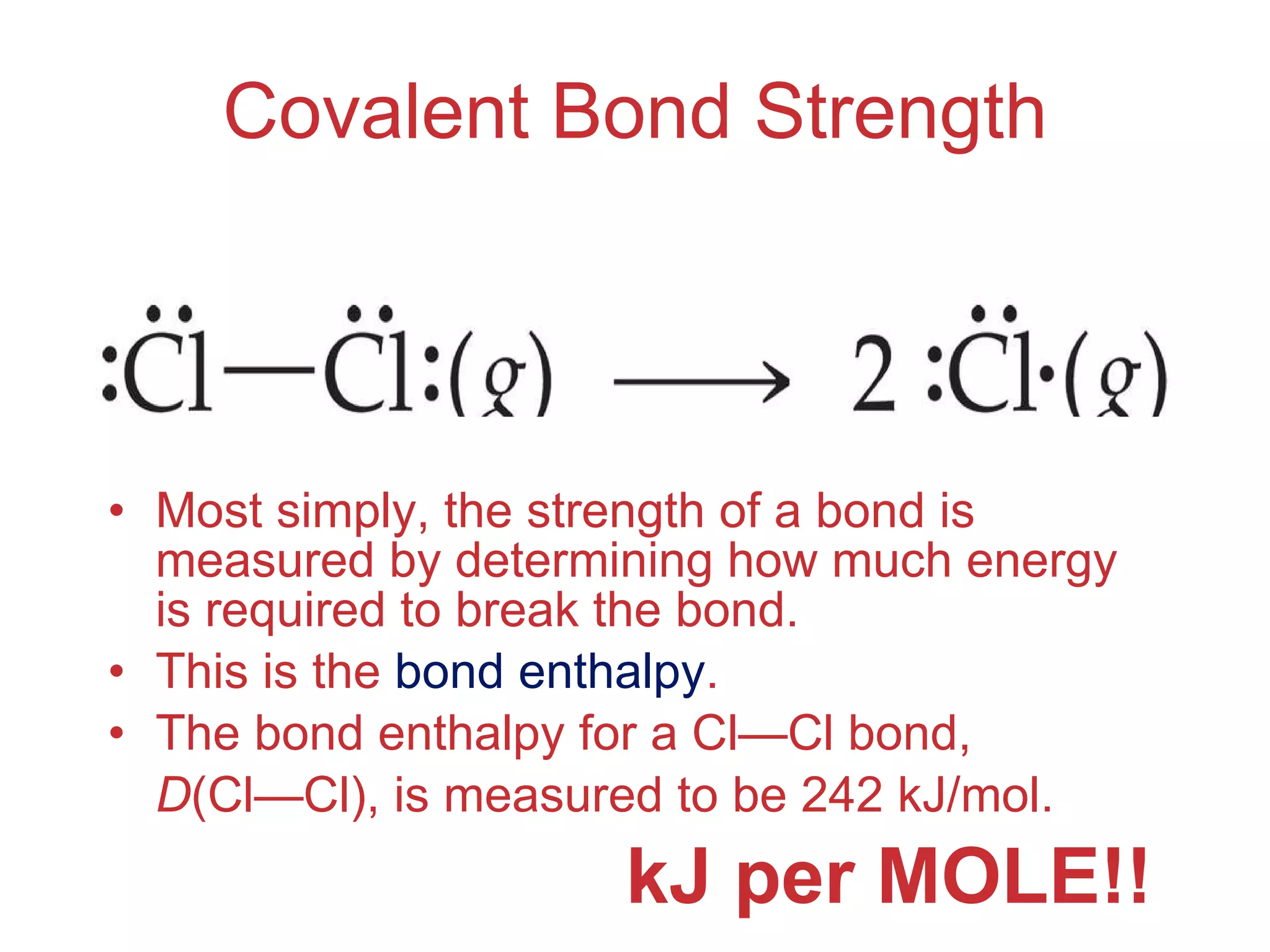
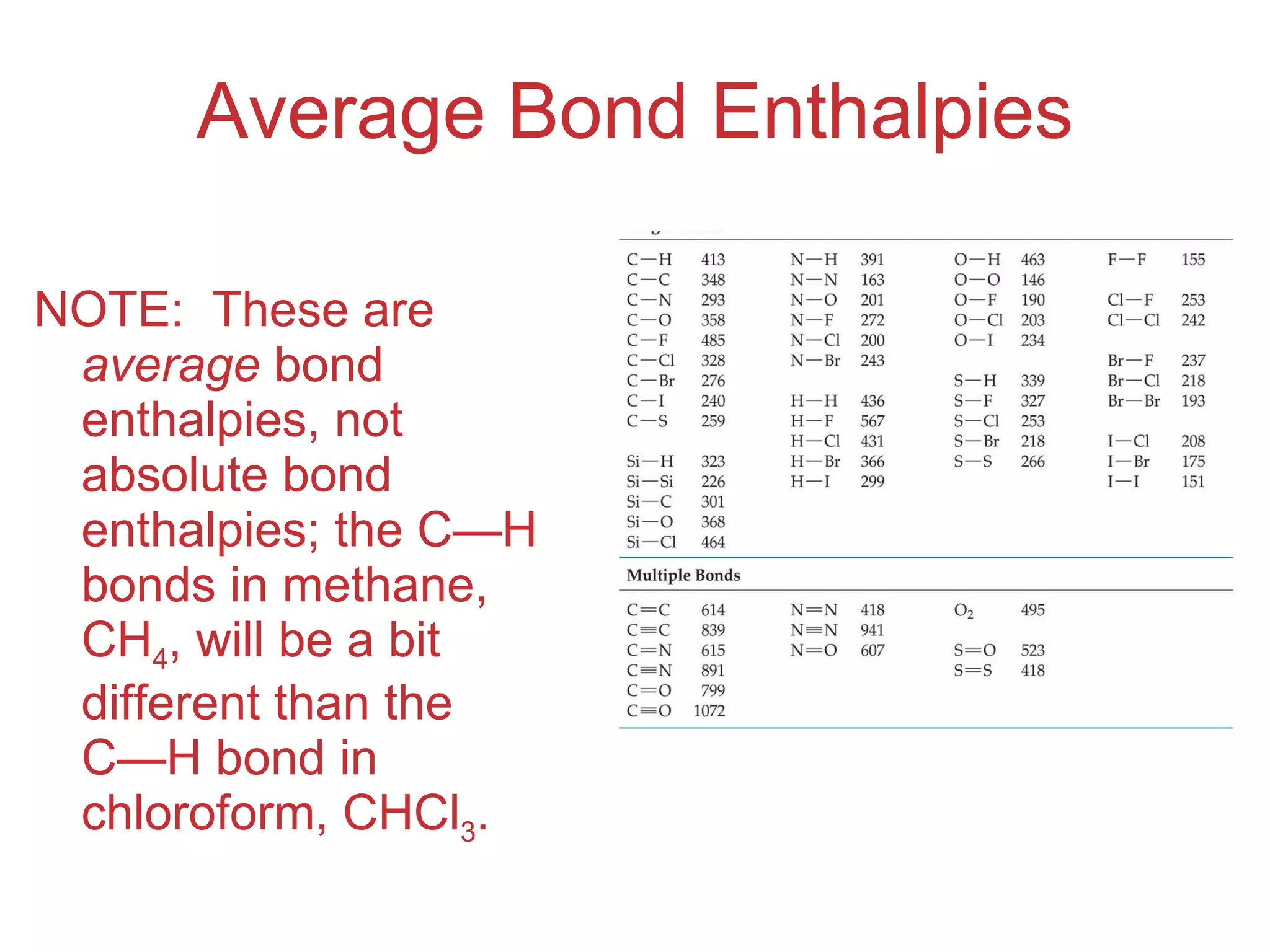
The document provides information about chemical bonds including ionic bonds, covalent bonds, and bond energies. It defines ionic and covalent bonding, discusses factors that determine lattice energy of ionic compounds, introduces electronegativity and bond polarity. It also covers Lewis structures, resonance structures, and exceptions to the octet rule. Bond enthalpies, which measure bond strength, are discussed along with average bond enthalpies from bond dissociation data.






























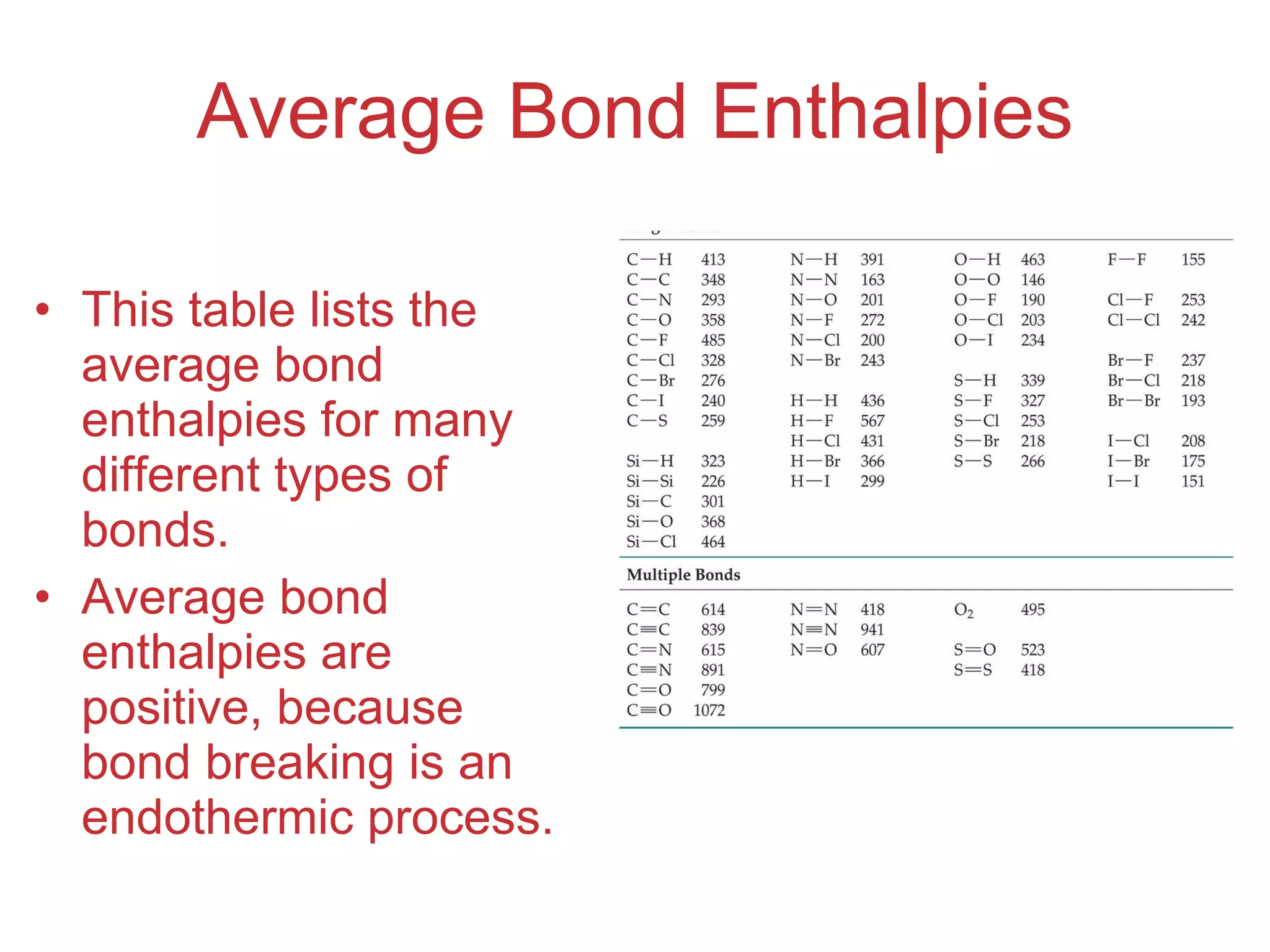
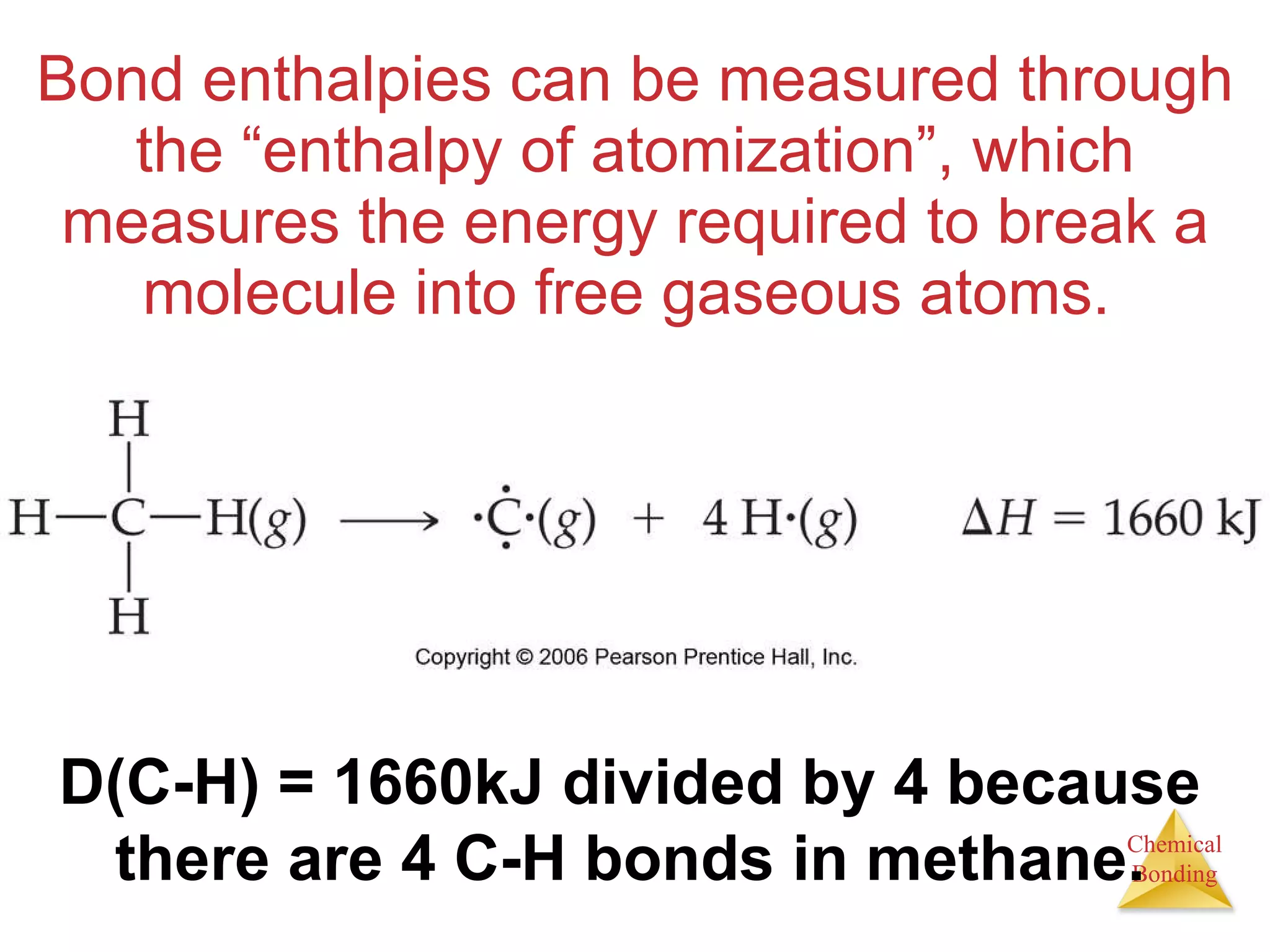

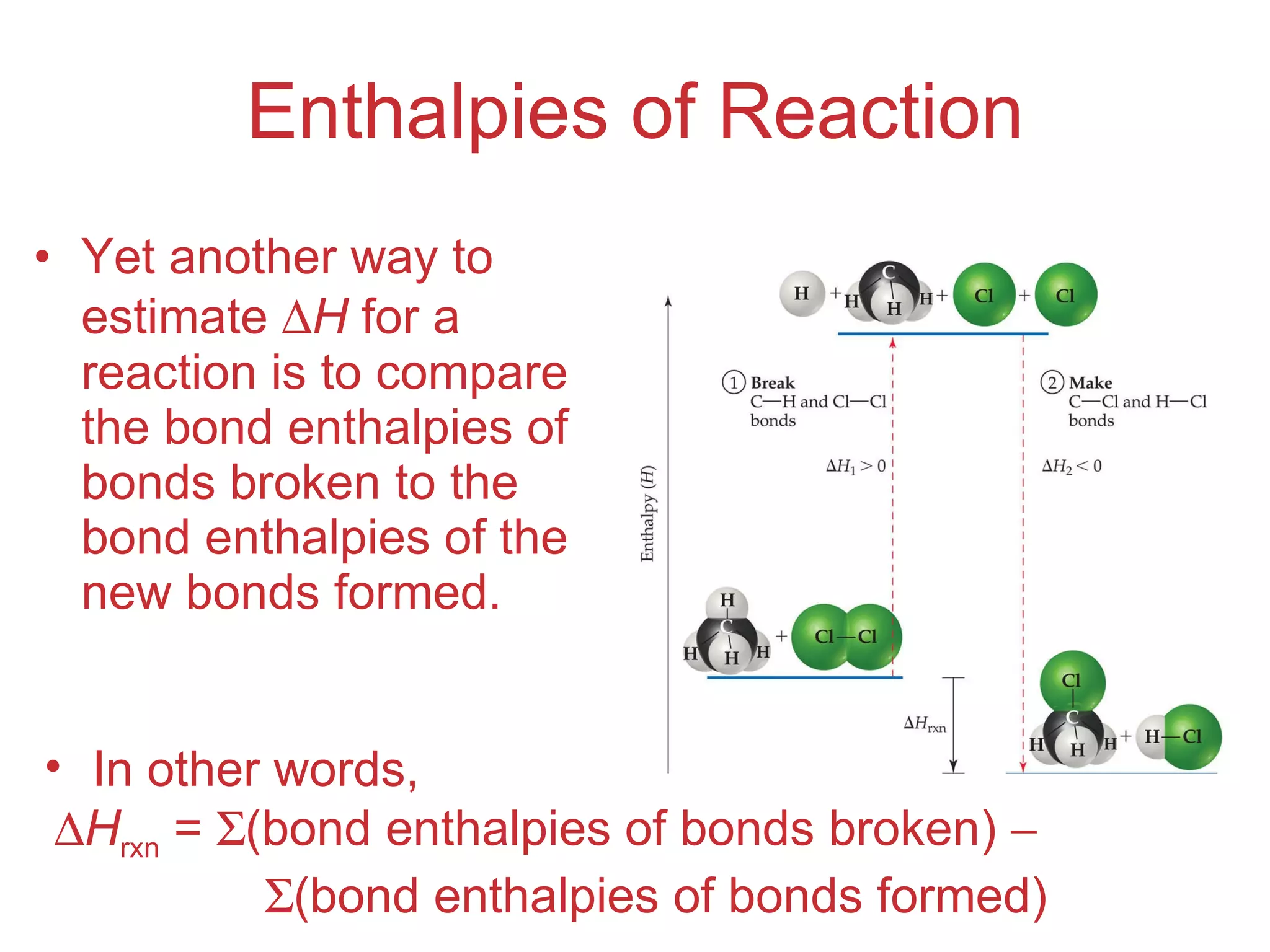
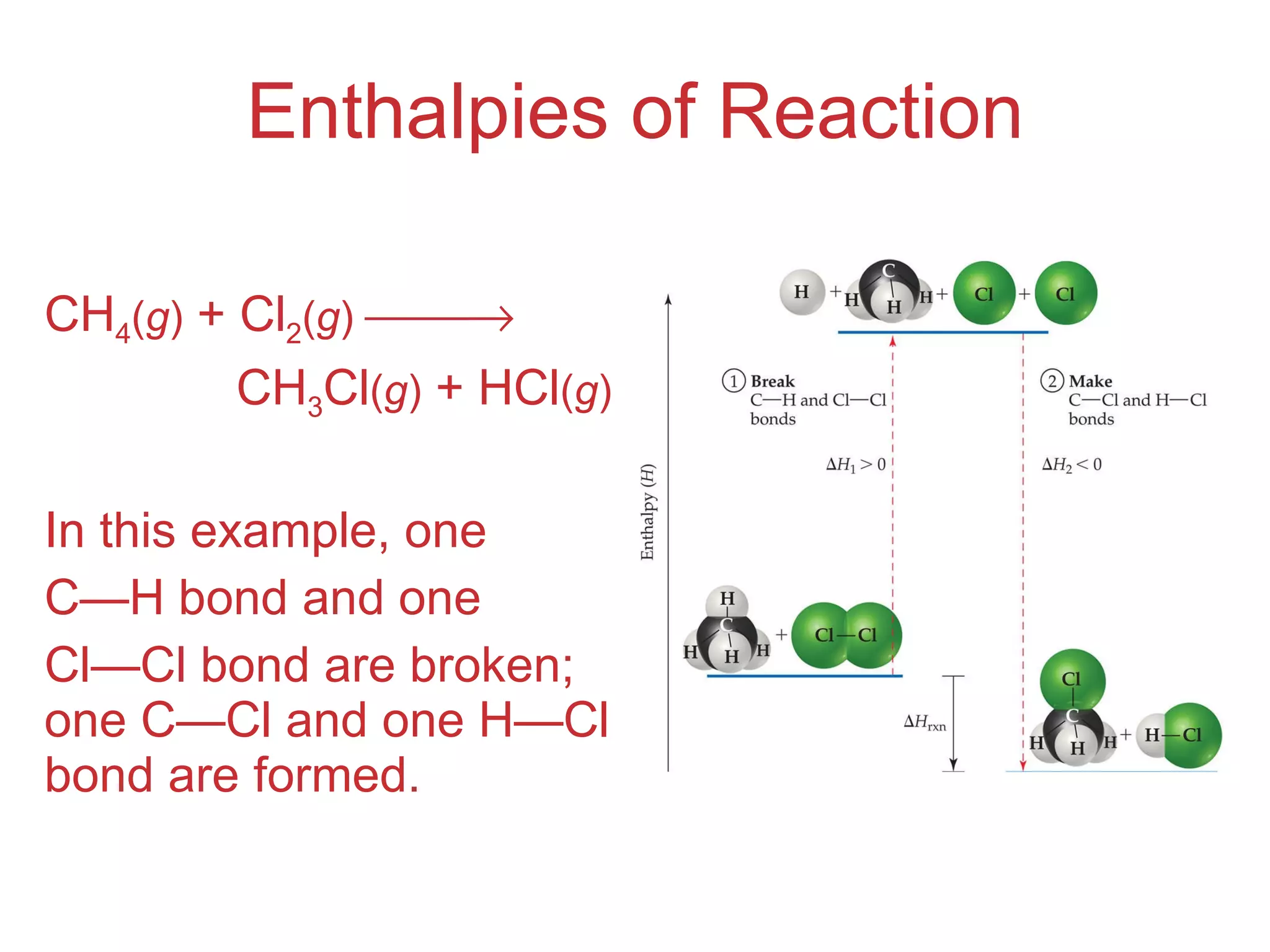
![Enthalpies of Reaction So, H rxn = [ D (C — H) + D (Cl — Cl) [ D (C — Cl) + D (H — Cl) = [(413 kJ) + (242 kJ)] [(328 kJ) + (431 kJ)] = (655 kJ) (759 kJ) = 104 kJ](https://image.slidesharecdn.com/8ap-091029071107-phpapp02/75/Chapter-8-Lecture-Basic-Bonding-93-2048.jpg)
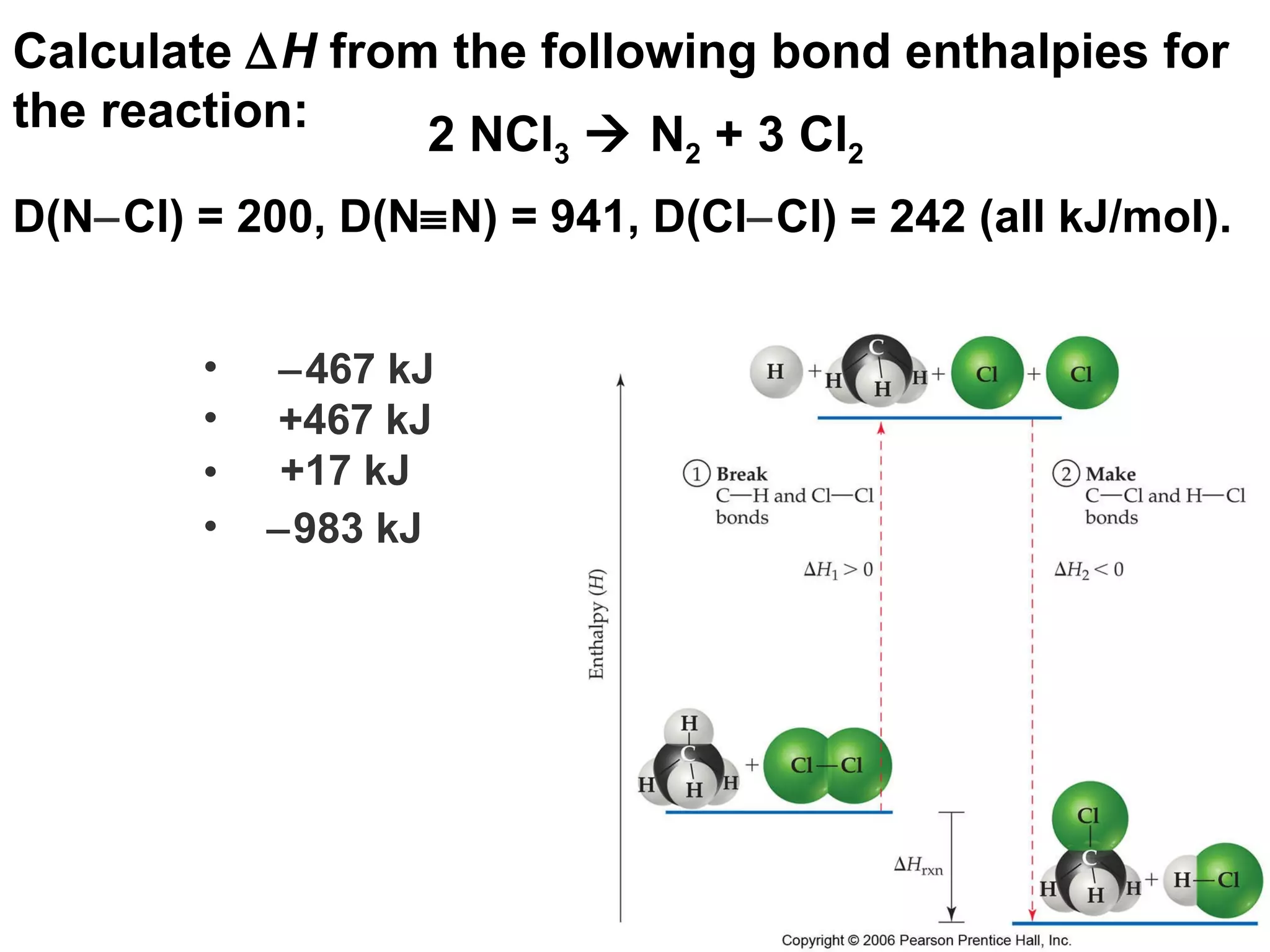
![Correct Answer: H = (bond enthalpies of bonds broken) ( bond enthalpies of bonds formed) H = 6(200) [941 + 3(242)] H = 1200 (1667) H = 467 467 kJ +467 kJ +17 kJ 983 kJ](https://image.slidesharecdn.com/8ap-091029071107-phpapp02/75/Chapter-8-Lecture-Basic-Bonding-96-2048.jpg)