Embed presentation
Download to read offline

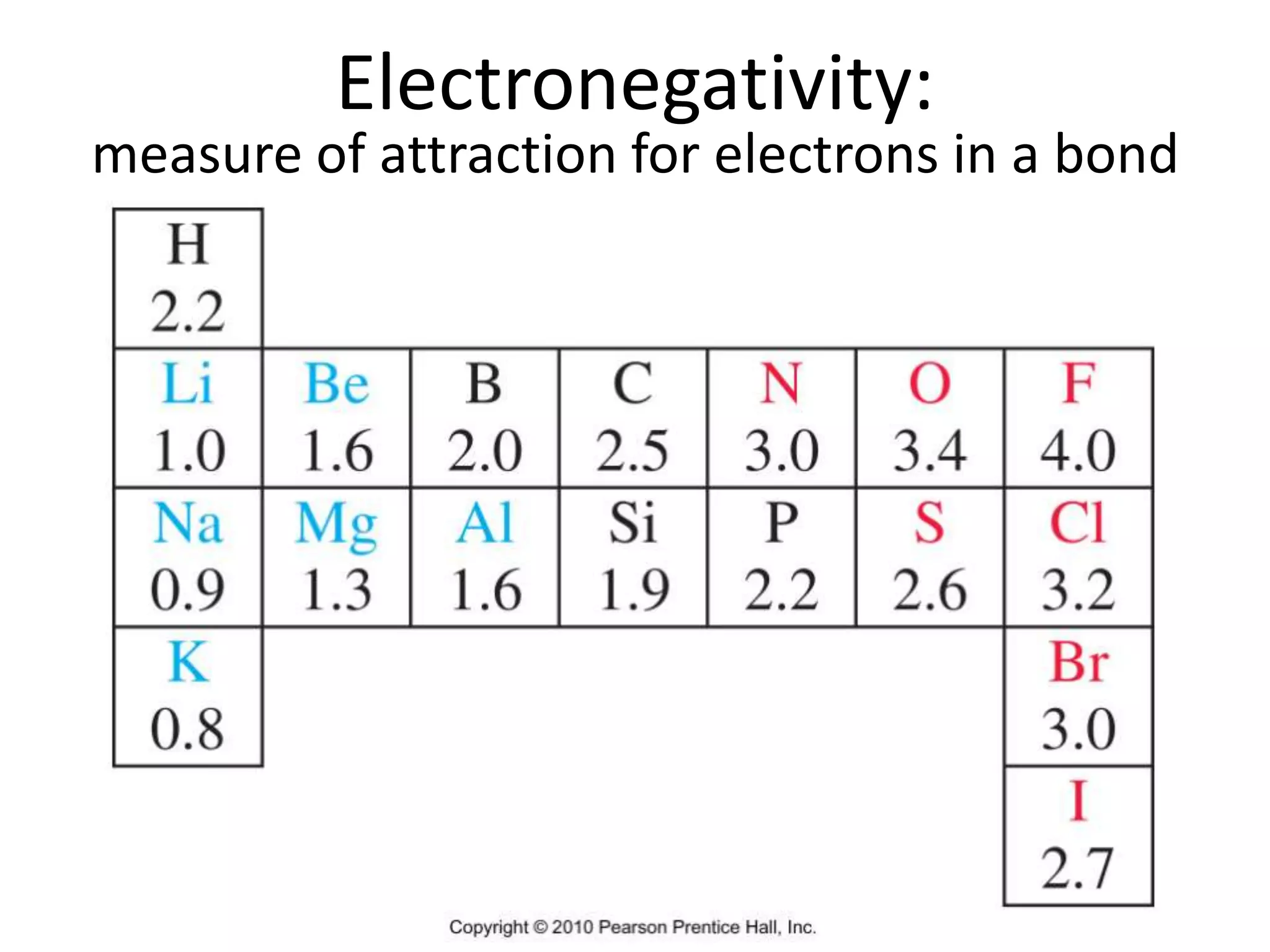
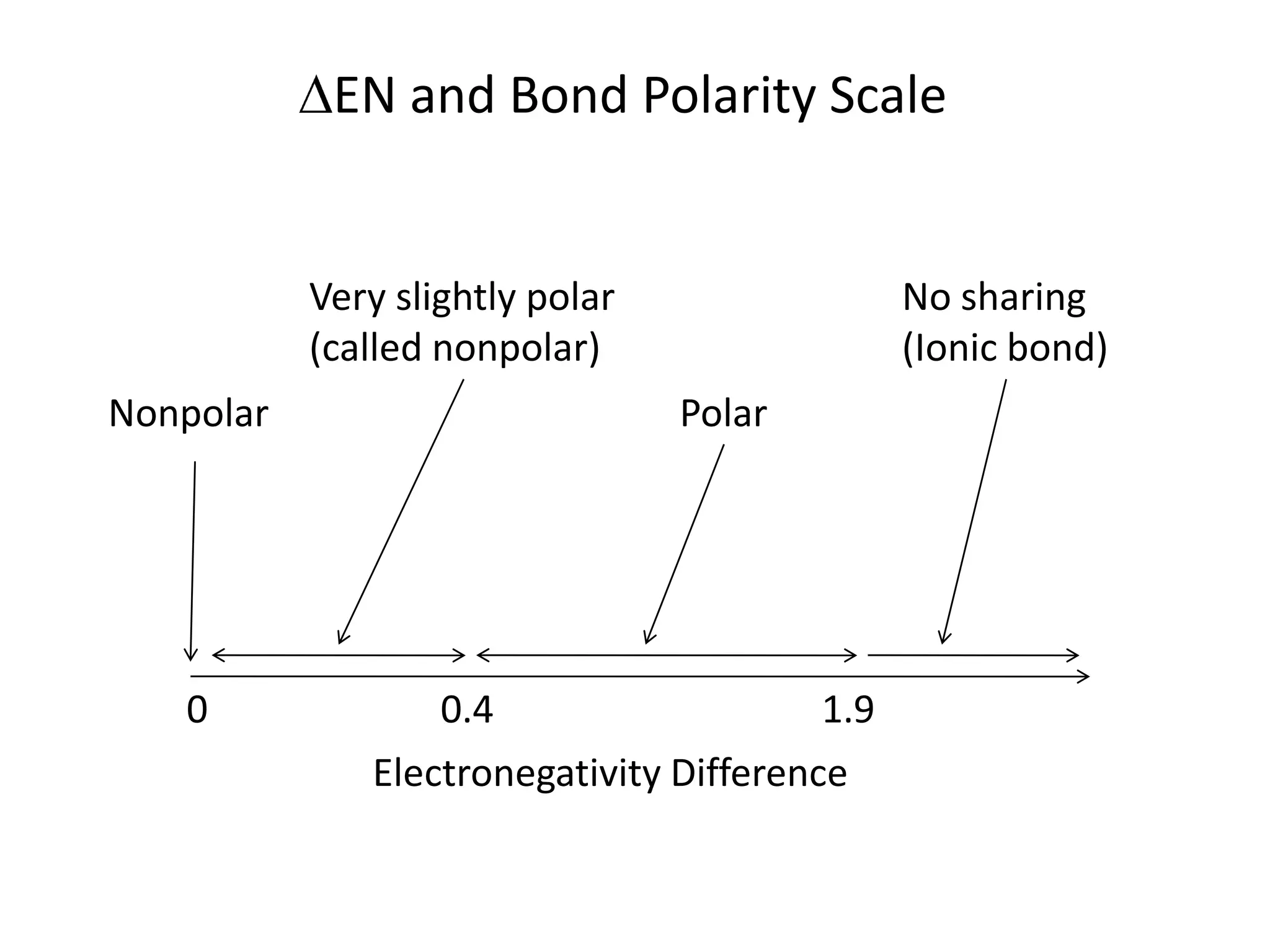
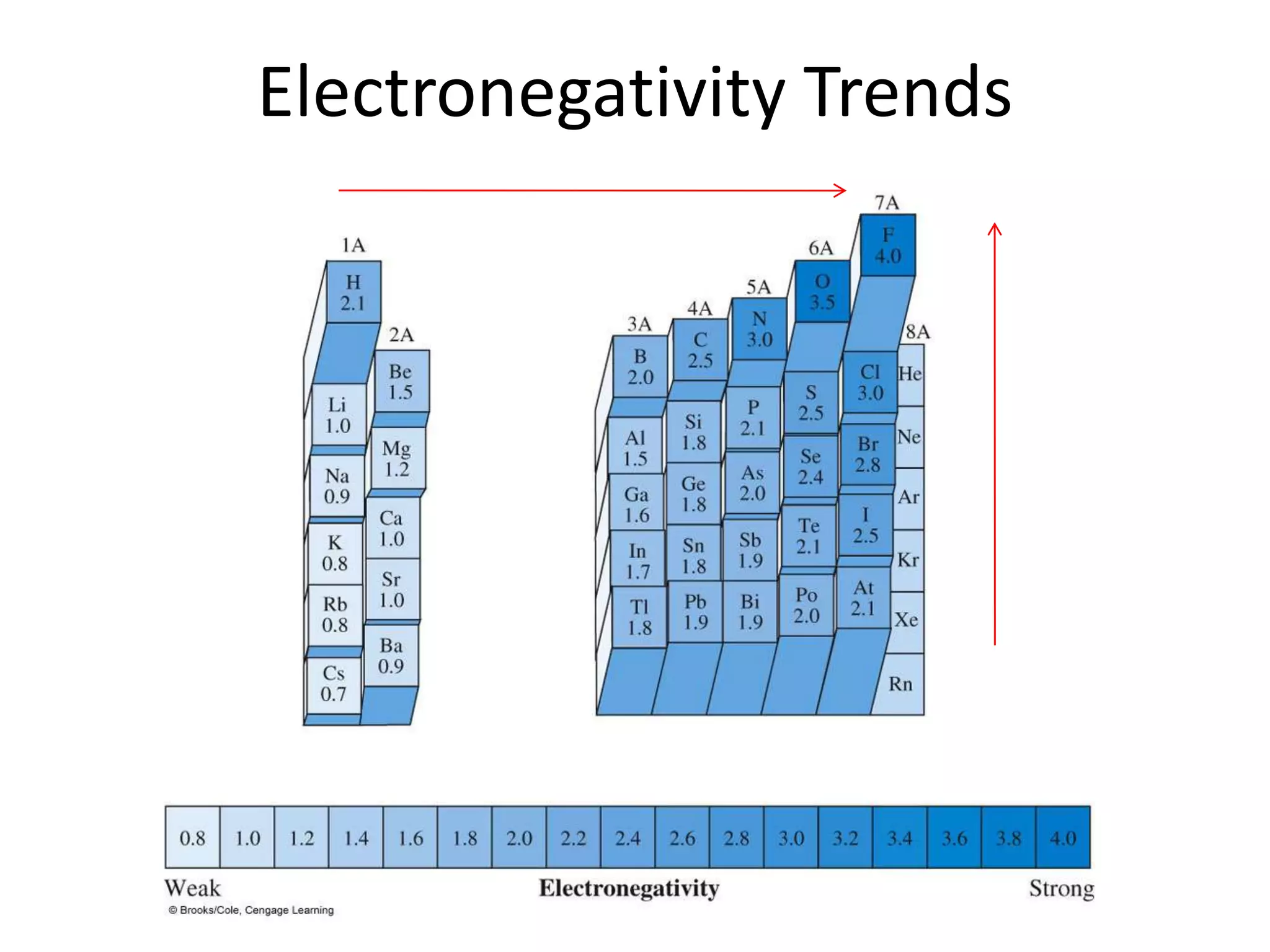
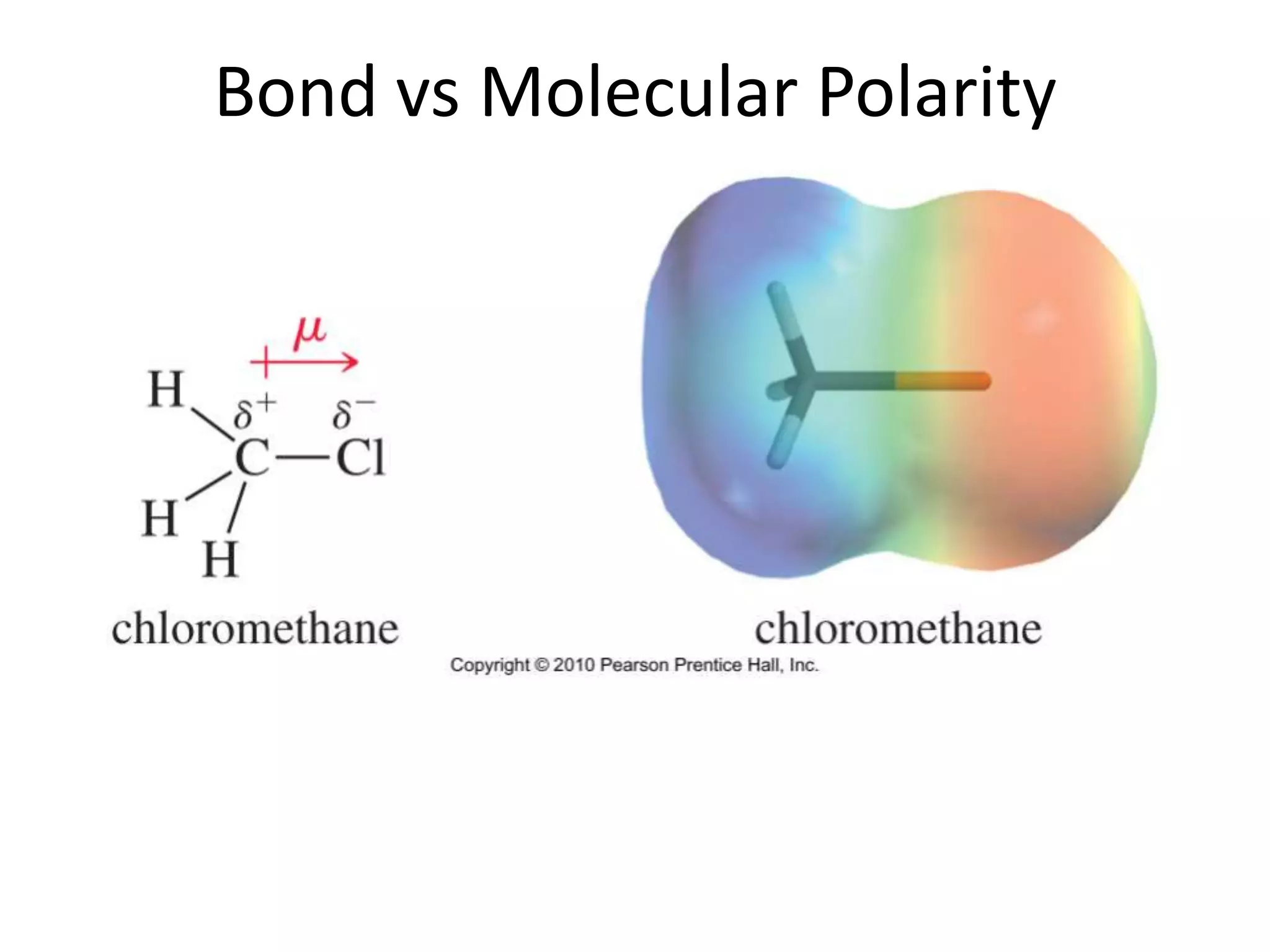
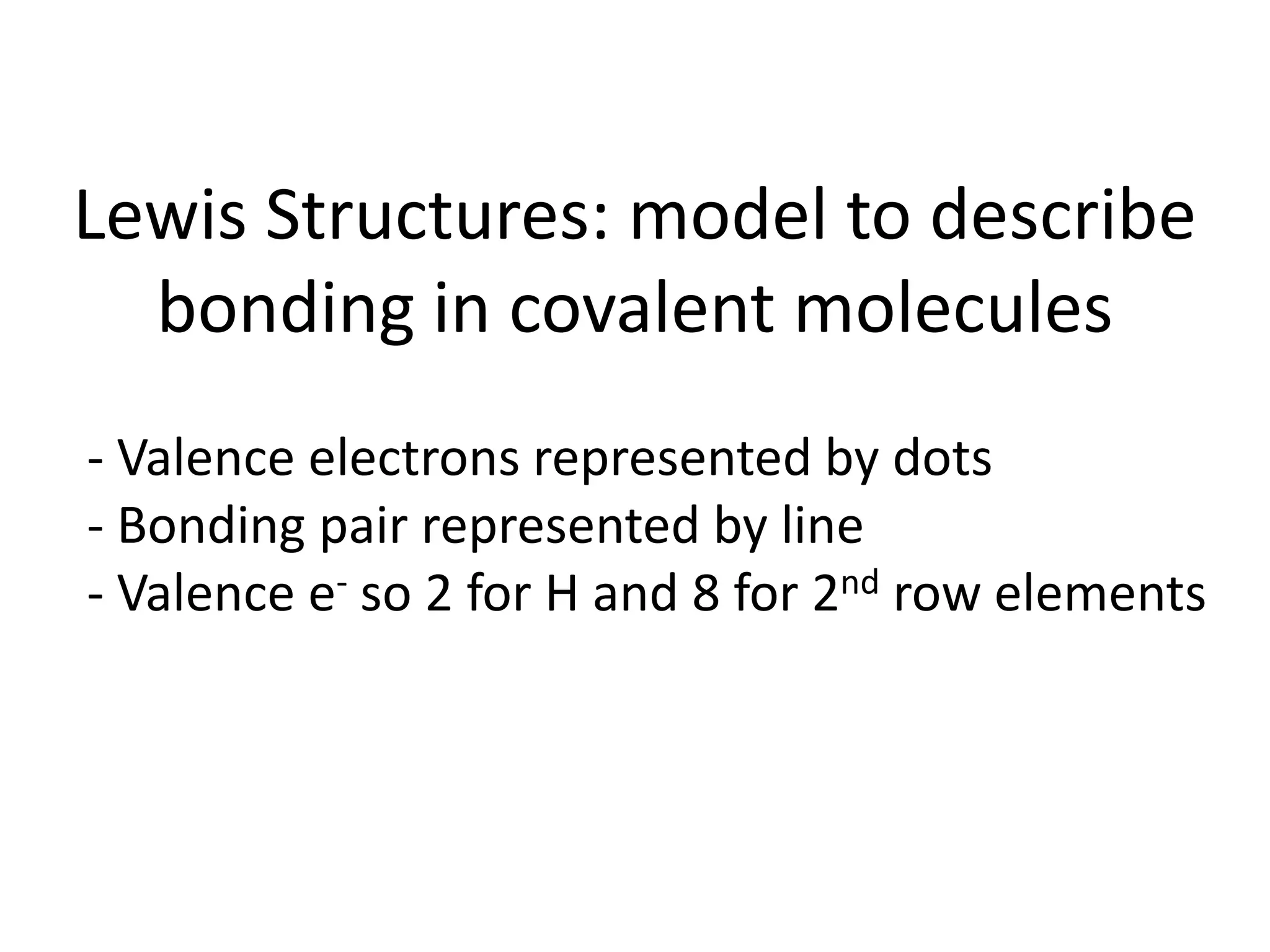
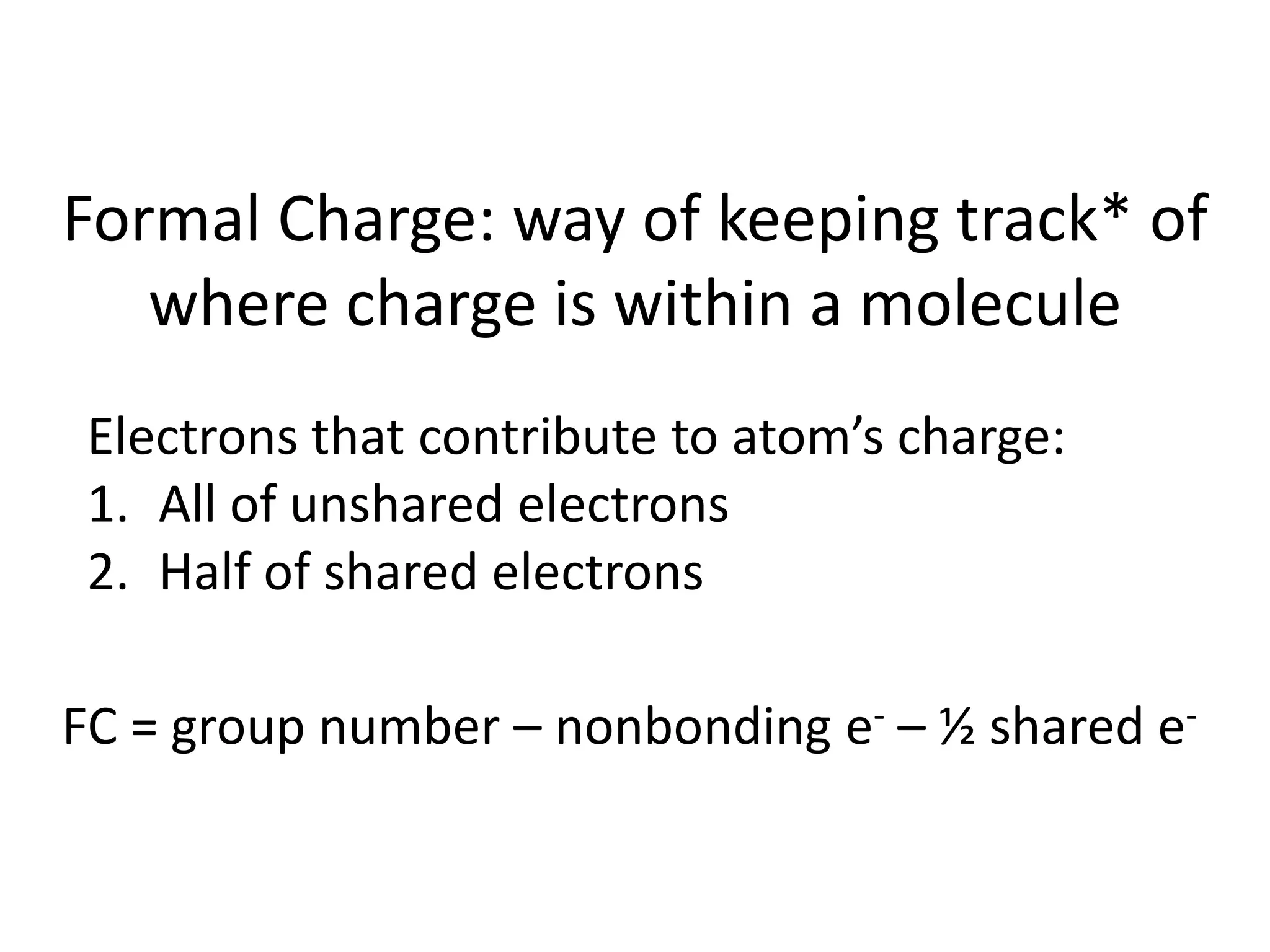


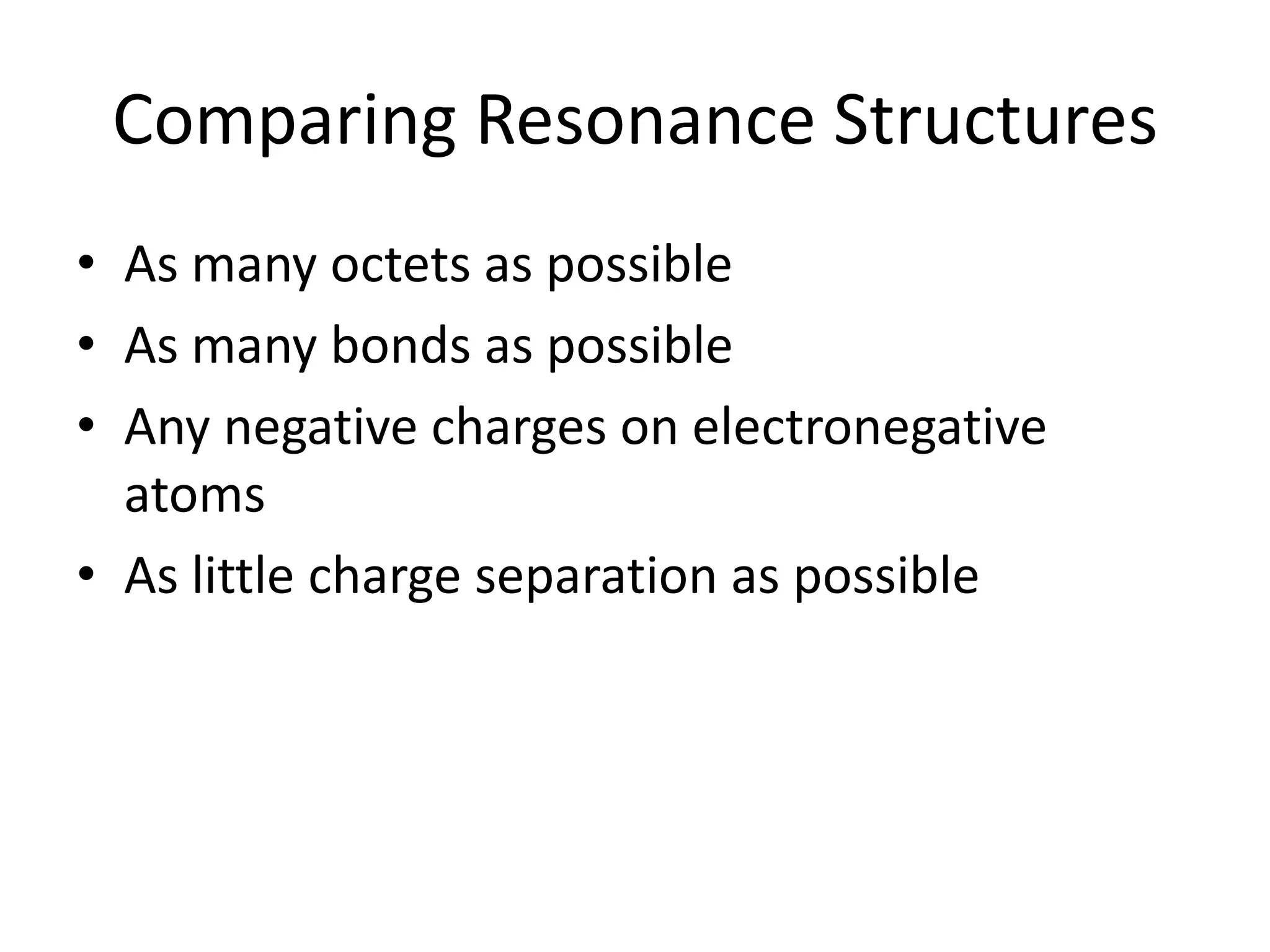
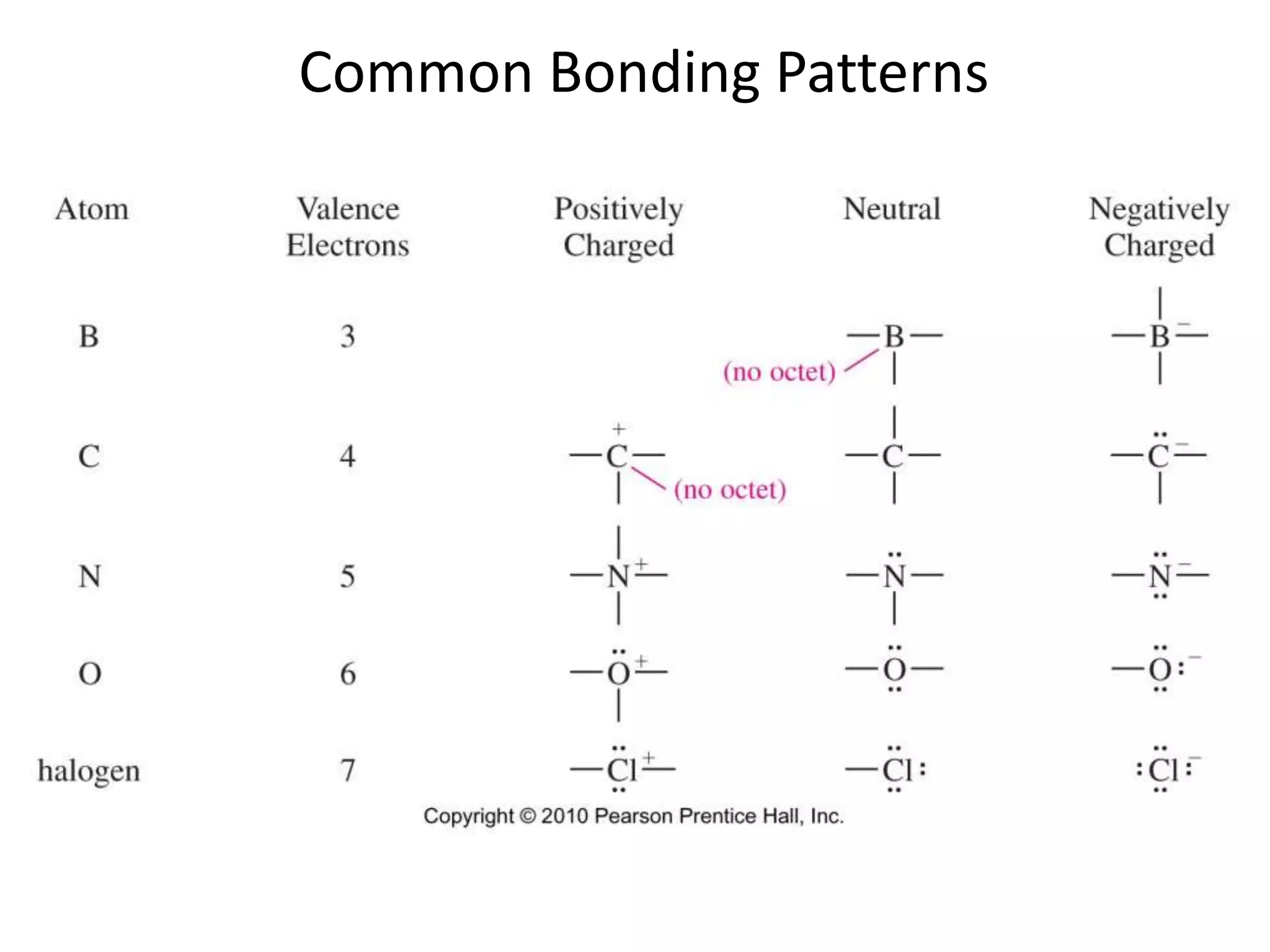
This document discusses bonding types, including ionic and covalent bonding. It introduces Lewis structures as a model to represent covalent bonding using dots to represent valence electrons and lines for bonding pairs. Formal charge is defined as a way to track the distribution of charge within a molecule based on an atom's group number and bonded/nonbonded electrons. Resonance structures are discussed as an extension of Lewis structures to account for delocalized charge.











