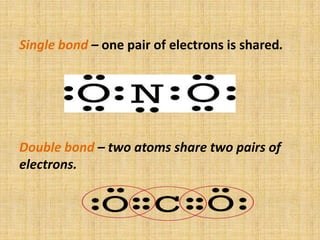
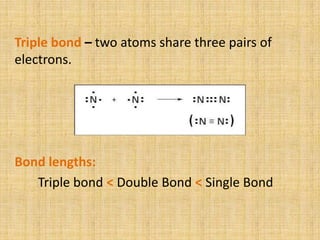
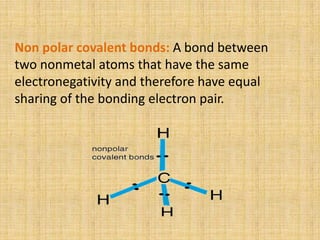
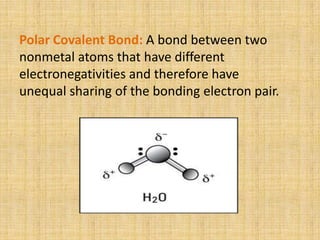
Covalent bonds form between non-metal atoms by sharing electron pairs. There are three main types of covalent bonds: single, double, and triple bonds which share 1, 2, or 3 electron pairs respectively. Covalent bonds can be nonpolar, with equal electron sharing between atoms of the same electronegativity, or polar, with unequal sharing between atoms of different electronegativities. Compounds with covalent bonds exist as gases, liquids, or solids and have properties such as being bad conductors and soluble in non-polar rather than polar solvents.