Process flow for non sterile and sterile manufacturing process
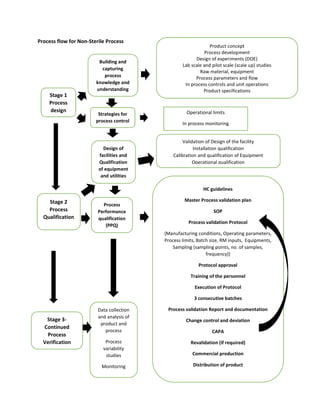
- 1. Process flow for Non-Sterile Process Stage 1 Process design HC guidelines Master Process validation plan SOP Process validation Protocol {Manufacturing conditions, Operating parameters, Process limits, Batch size, RM inputs, Equipments, Sampling (sampling points, no. of samples, frequency)} Protocol approval Training of the personnel Execution of Protocol 3 consecutive batches Process validation Report and documentation Change control and deviation CAPA Revalidation (if required) Commercial production Distribution of product Strategies for process control Building and capturing process knowledge and understanding Stage 3- Continued Process Verification Validation of Design of the facility Installation qualification Calibration and qualification of Equipment Operational qualification Stage 2 Process Qualification Product concept Process development Design of experiments (DOE) Lab scale and pilot scale (scale up) studies Raw material, equipment Process parameters and flow In process controls and unit operations Product specifications Design of facilities and Qualification of equipment and utilities Process Performance qualification (PPQ) Data collection and analysis of product and process Process variability studies Monitoring Operational limits In process monitoring
- 2. Process flow for Sterile Process Stage 1 Sterile Process design HC guidelines Master sterile Process validation plan SOP Process validation Protocol {Manufacturing conditions, Operating parameters, Process limits, Batch size, RM inputs Equipments, Sampling (sampling points, no. of samples, frequency)} Protocol approval Training of the personnel Execution of Protocol Process Simulation (start up, processing operations, aseptic connections, filling, closing) Microbiological media Worst 3 batches (Incubation Time – 14 to 21 days., Fill Volume, Analytical tests and evaluation for microbial growth, No. of units manufactured, Personnel Frequency of testing, Raw material quality, Packaging material testing requirements.) Process validation Report and documentation Change control and deviation CAPA Revalidation (if required) Commercial production Distribution of product Strategies for process control Building and capturing process knowledge and understanding Stage 3- Continued Process Verification Validation of Design of the facility Installation qualification Calibration and qualification of Equipment Operational qualification Clean room setup, qualified and controls in place EM and analytical method validated Stage 2 Process Qualification Sterile Product concept Sterile Process development Design of experiments (DOE) Lab scale and pilot scale (scale up) studies Raw material, equipment Process parameters and flow Sterile In-process controls and unit operations Product specifications Design of facilities and Qualification of equipment and utilities Process Performance qualification (PPQ) Data collection and analysis of product and process Process variability studies Monitoring Operational limits In process monitoring
