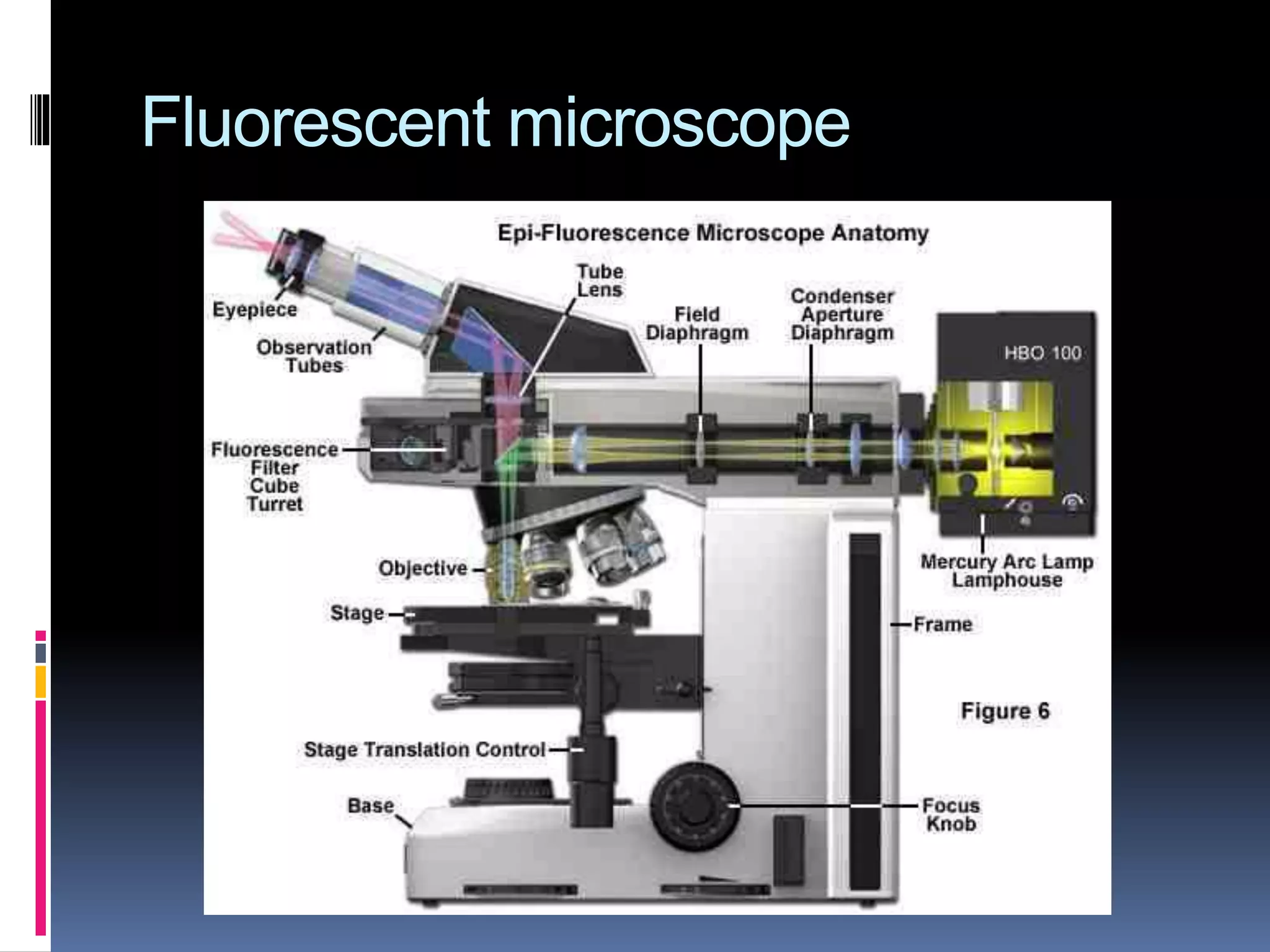
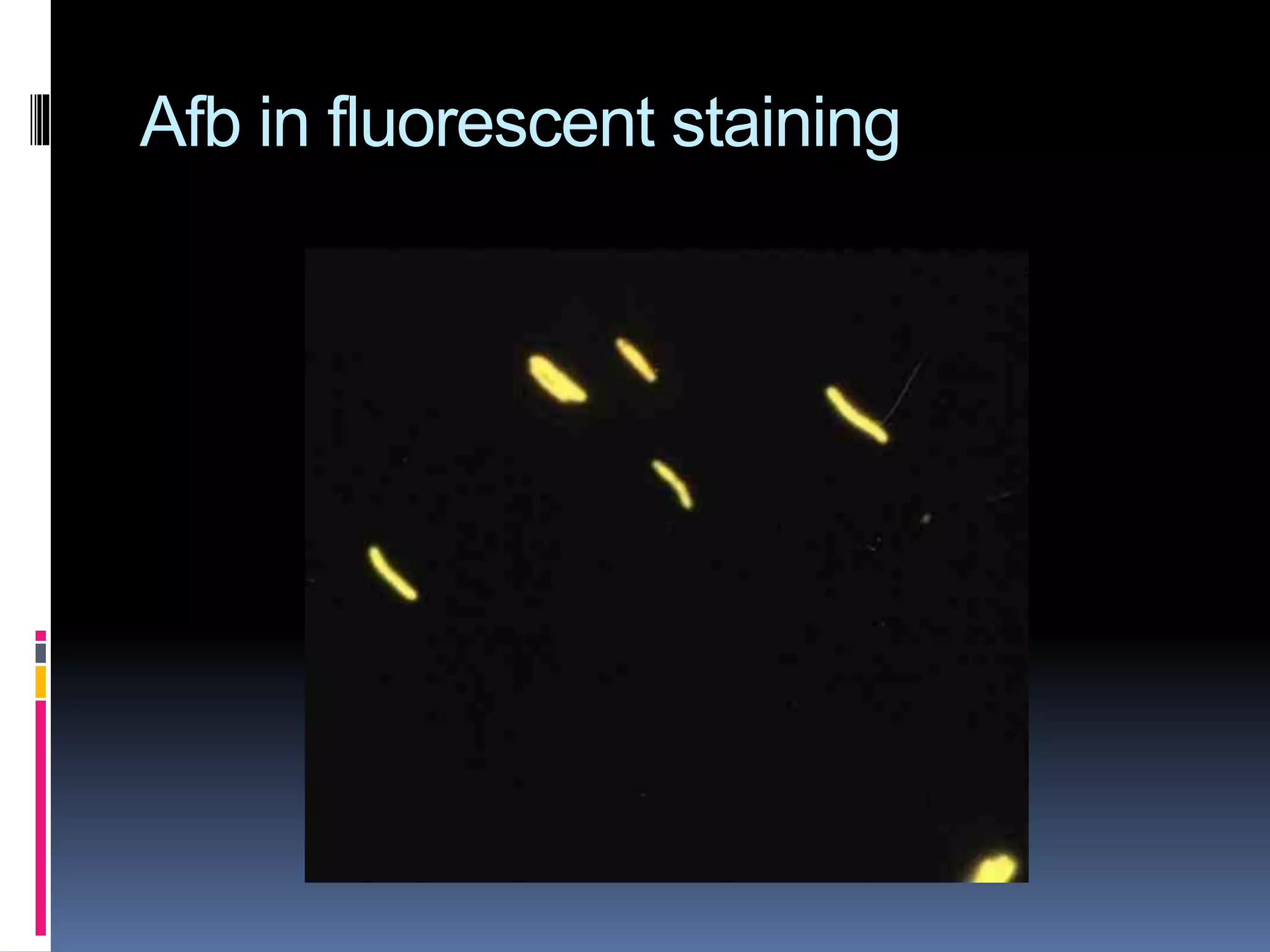
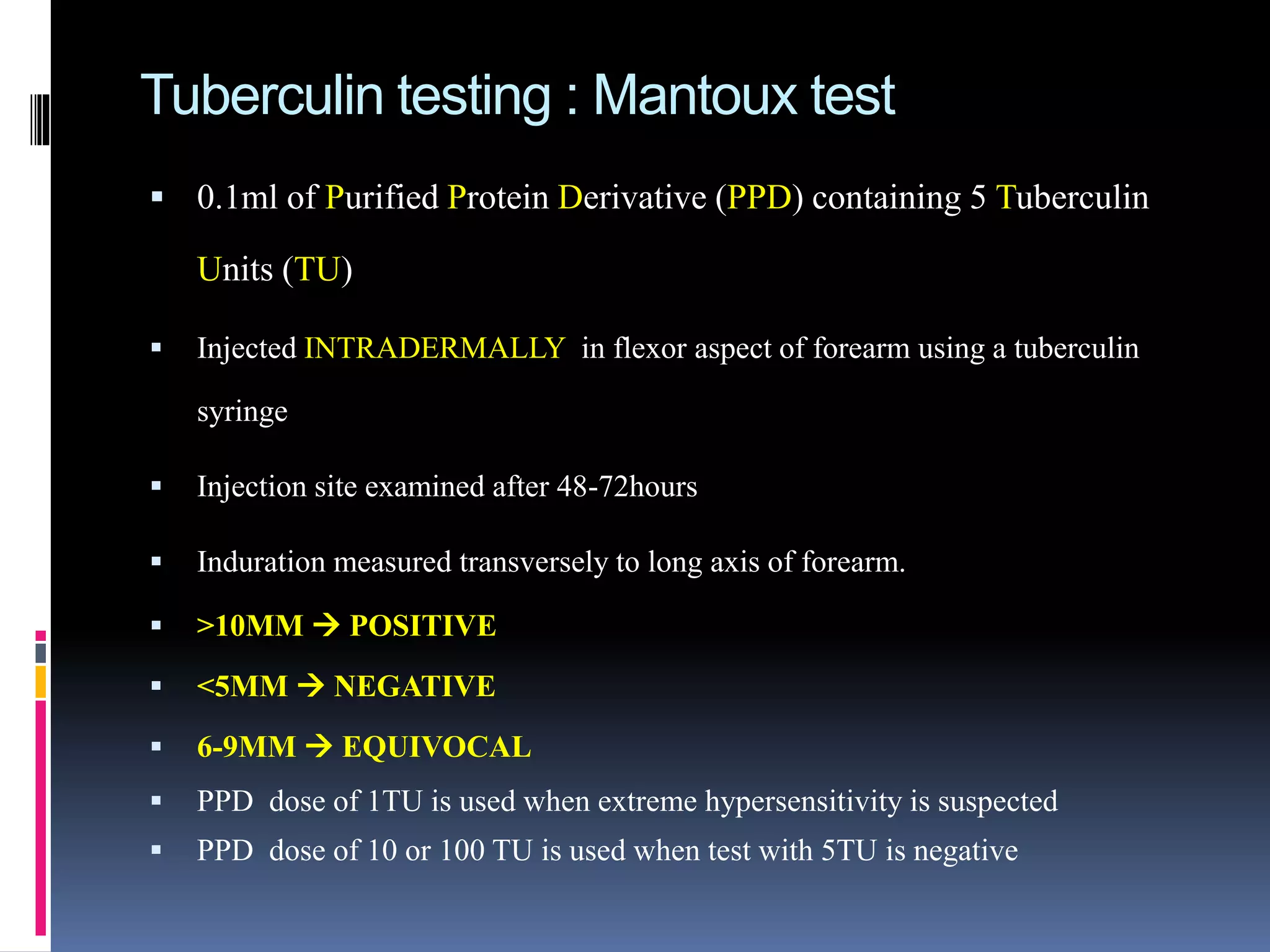
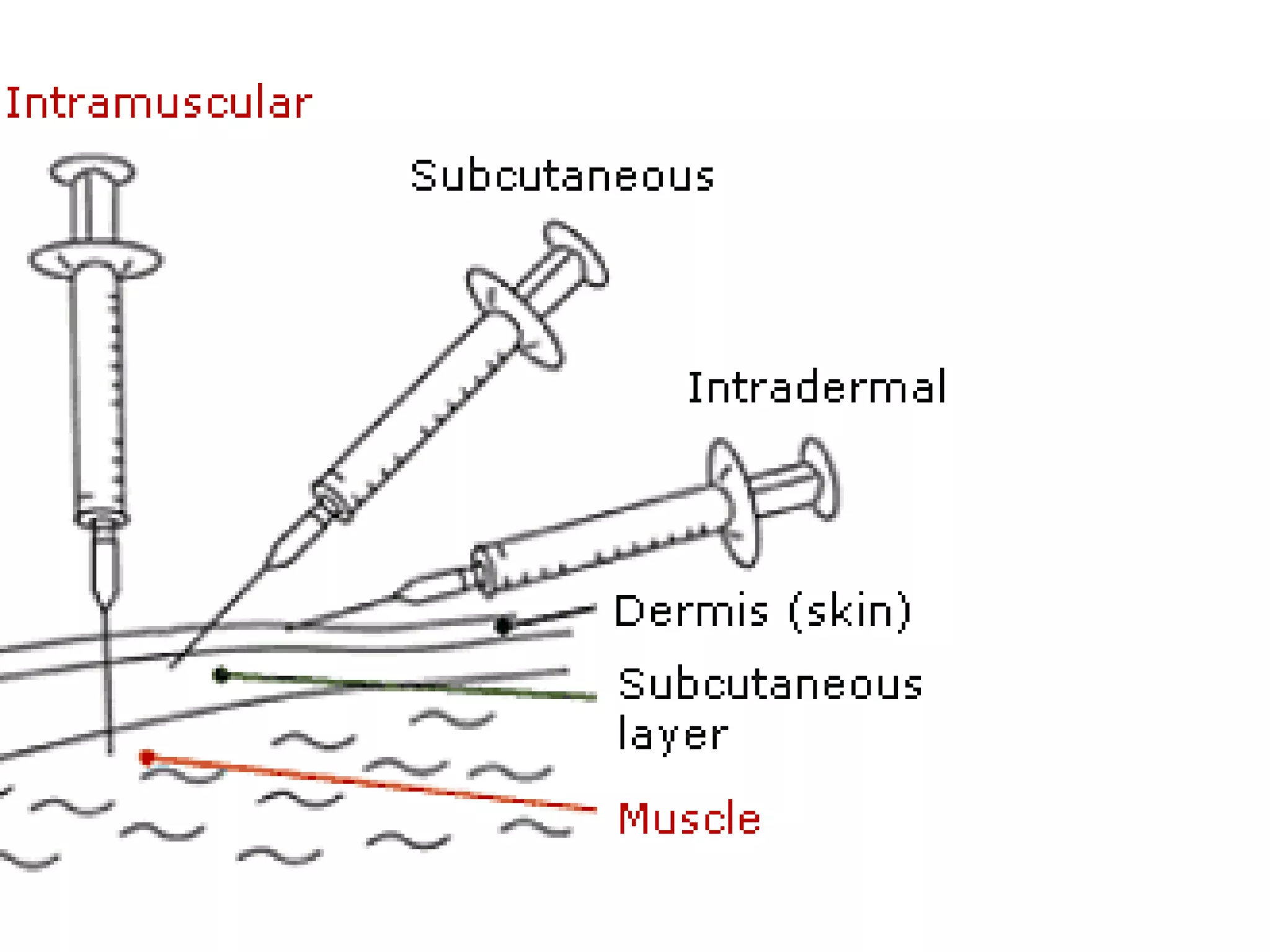
This document provides information on the diagnosis and treatment of tuberculosis. It discusses methods for collecting and analyzing sputum samples under microscopy and culture to identify Mycobacterium tuberculosis. It also summarizes techniques for assessing drug resistance and extra-pulmonary tuberculosis diagnosis. Treatment involves short course multidrug regimens and directly observed therapy programs.