The Hydrogen Atom Wave Function Directly Observed
- 1. From First Principles PART V – THE HYDROGEN ATOM June 2017 – R3.4 Maurice R. TREMBLAY
- 2. Prolog What you’re looking at is the first direct observation of an atom’s electron orbital — an atom’s actual wave function! Up until this point, scientists have never been able to actually observe the wave function. Trying to catch a glimpse of an atom’s exact position or the momentum of its lone electron has been like trying to catch a swarm of flies with one hand. [c.f. Phys. Rev. Lett. 110, 213001 (2017)] 2
- 3. Photoionization microscopy can directly obtain the nodal structure of the electronic orbital of a Hydrogen atom placed in a static electric field. In the experiment, the Hydrogen atom is placed in the electric field E and is excited by laser pulses. The ionized electron escapes from the atom and follows a particular tra- jectory to the detector that is perpendicular to the field itself. Given that there are many such trajectories that reach the same point on the detector, interference patterns can be observed […]. The interference pattern directly reflects the nodal structure of the wavefunction. [c.f. Phys. Rev. Lett. 110, 213001 (2017)] Calculated Measured 3
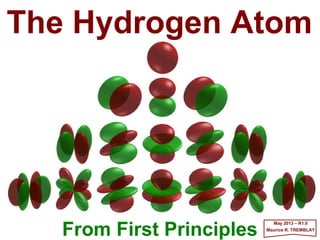
- 4. n = 1 llll = 0 mllll = 0 n = 2 llll = 0 mllll = 0 ↑↑↑↑ z n = 2 llll = 1 mllll = ±±±±1 n = 2 llll = 1 mllll = 0 n = 3 llll = 1 mllll = ±±±±1 n = 3 llll = 1 mllll = 0 n = 3 llll = 0 mllll = 0 n = 3 llll = 2 mllll = ±±±±1 n = 3 llll = 2 mllll = 0 n = 3 llll = 2 mllll = ±±±±2 Contents PART V – THE HYDROGEN ATOM What happens at 10−−−−10 m? The Hydrogen Atom Spin-Orbit Coupling Other Interactions Magnetic & Electric Fields Hyperfine Interactions Multi-Electron Atoms and Molecules Appendix – Interactions The Harmonic Oscillator Electromagnetic Interactions Quantization of the Radiation Field Transition Probabilities Einstein’s Coefficients Planck’s Law A Note on Line Broadening The Photoelectric Effect Higher Order Electromagnetic Interactions References Probability patterns for the electron in the Hydrogen atom. The computer generated pictures show slices through the atom for the lowest energy levels. The pictures are coded so that the bright regions correspond to high probability of finding the electron. 2017 MRT “The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known, and the difficulty lies only in the fact that the exact application of these laws leads to equations much too complicated to be soluble.” Paul Dirac, ‘Quantum Mechanics of Many-Electron Systems’ Proceedings of the Royal Society (London),123 (1929) pp. 714-733. 4
- 5. 2017 MRT “In order to explain the results of experiments on scattering of αααα rays by matter Prof. Rutherford has given a theory of the structure of atoms. According to this theory, the atom consist of a positively charged nucleus surrounded by a system of electrons kept together by attractive forces from the nucleus; the total negative charge of the electrons is equal to the positive charge of the nucleus.” Niels Bohr, Philos. Mag. 26, 1 (1913). PART IV – QUANTUM FIELDS Review of Quantum Mechanics Galilean Invariance Lorentz Invariance The Relativity Principle Poincaré Transformations The Poincaré Algebra Lorentz Transformations Lorentz Invariant Scalar Klein-Gordon & Dirac One-Particle States Wigner’s Little Group Normalization Factor Mass Positive-Definite Boosts & Rotations Mass Zero The Klein-Gordon Equation The Dirac Equation References PART III – QUANTUM MECHANICS Introduction Symmetries and Probabilities Angular Momentum Quantum Behavior Postulates Quantum Angular Momentum Spherical Harmonics Spin Angular Momentum Total Angular Momentum Momentum Coupling General Propagator Free Particle Propagator Wave Packets Non-Relativistic Particle Appendix: Why Quantum? References 5
- 6. What happens at 10−−−−10 m? 2017 MRT This is the last of a three-part series on quantum mechanics. I initially intended to finish this work with a good description of what we think an atom such as Hydrogen looks like and show you in detail its atomic orbital framework and then end there but I deemed the content void of a few important features: the Harmonic Oscillator and an introduction to Electromagnetic Interactions which leads directly to a formulation of the Quantization of the Radiation Field! Then I could not finish without wrapping it up with a development of Transition Probabilities and Einstein Coefficients which opens up the proof of the Planck distribution law, the photoelectric effect and higher order electromagnetic interactions – in essence justifying how things started in PART III. I believe this is the key contribution: making it more understandable up to, but not including, Quantum Electrodynamics (QED)! For that QED part, you can refer to PART VII which is entirely dedicated to its formulation. On the other hand, I then felt compelled to add a whole new chapter on Multi-Electron Atoms and Molecules which I have taken almost piecemeal from the book from David B. Beard and George B. Beard, Quantum Mechanics with Applications. 6 Now, its funny but all of this rough work of presenting all this machinery of quantum mechanics, angular momentum and Dirac’s equation leads us now to the exercise of formulating the Schrödinger equation with a non-vanishing radial potential V(r) which is the Coulomb potential between the Hydrogen atom’s nucleus (i.e., the proton) and an electron in ‘orbit’, &c. But we will do better by taking the v2/c2 approximation to Dirac’s equation developed earlier in PART IV and just limit ourselves here to only a few cases of academic and theoretical interest notably Spin-Orbit Coupling and Other Interactions such as Magnetic & Electric Fields and Hyperfine Interactions.
- 7. 2017 MRT As I will mention in the Multi-Electron Atoms and Molecules chapter, only reasonably accurate solutions of the Hydrogen atom are possible because it is comprised of only one electron surrounding a single proton and Bohr radius of the electron is ~0.5×10−10 m. I say ‘reasonably accurate solutions’ because the differential equations that indepen- dently represent the radial, angular and azimuthal coordinate functions are solved pretty much only by assuming a series expansion. So, in effect, for the simplest kind of atom in the universe, mankind still hasn’t figured out an exact solution to the differential equa- tions that represent its “orbitals”… Now, Helium atoms are composed of a nucleus com- posed on two protons and two neutrons (i.e., an alpha particle) that is surrounded by two electrons. In theory, this is a three-body problem; it cannot be solved explicitly in closed form as unfortunately no one has ever been able to solve in closed form any three-dimen- sional case involving more than two interacting particles. However, as with the Hydrogen atom, approximate solutions can be obtained for the energy of the ground state using perturbation theory and I will show you how to do it but only up to first-order accuracy. 7 In essence, it is so important to understand one thing: the quantum ‘mechanics’ used in this theory of the representation of the Hydrogen atom has generated vast amounts of quantitative verifications – even relativistically! So, this is the way nature is like to a certain degree since I did not include interactions with other particles or photons. Nature does act weird at small scales, i.e., those of the atom or about 10−10 m! An electron does have spin and it does fill defined orbitals which can be represented quite faithfully for a Hydrogen atom – one of the simplest, yet quite intricate solution. This is the outcome of quantum mechanics – allowing us to finally ‘see’ an atom! Well, a kinda blury one!
- 8. 2017 MRT By weight, 75% of the visible* universe is Hydrogen, a colorless gas. In space, vast quantities interact with starlight to create spectacular sights such as the Eagle Nebula (seen by the Hubble Space Telescope). The Hydrogen atom is the simplest atom… yet: Symbol: H – element number 1. Atomic Weight: 1.008. Color: Colorless. Discovery: 1766 in the United Kingdom. Electron Configuration: 1 s 1 ; Valence: 1. Electronegativity: 2.2. Electron Affinity: 72.8 kJ/mol. Ionization Energies: 1312 kJ/mol. Atomic Radius: 53 pm; Covalent Radius: 37 pm; Van der Waals Radius: 120 pm. Crystal Structure: Simple Hexagonal. Density: 0.0899 g/l. Melting Point: −259.14 °C . Boiling Point: −252.87 °C . Thermal Conductivity: 0.1805 W/(m K). Gas phase: Diatomic. Magnetic Type: Diamagnetic. Mass Magnetic Susceptibility: −2.48×10 −8 . % in Universe: 75%; % in Sun: 75%; % in Meteorites: 2.4%; % in Earth's Crust: 0.15%; % in Oceans: 11%; % in Humans: 10%. Half-Life: Stable; Quantum Numbers: 2 S1/2. Stable Isotopes: 1 H, 2 H. Isotopic Abundances: 1 H - 99.9885% 2 H - 0.0115% The Hydrogen Atom 8 * In the form of baryonic mass and note that most of the universe’s mass is not in the form of baryons or chemical elements (e.g., dark matter and dark energy.)
- 9. with mo≡me the rest mass of the electron. By replacing E′ by E (non-relativistic energy); eφ by −V,and p by −ih∇∇∇∇, the above equation becomes the static Schrödinger equation: For an electron in a static field whose potential is ϕ, the Dirac equation (i.e., (E′+eϕ)ψ ={(1/2mo)[p+(e/c)A]2 +SMM[µS] Interaction−RE[p]−DT[p2]−SO[L••••S] Interaction}ψ – c.f., PART IV) without the higher-order relativistic corrections, simplifies and reduces to: 2017 MRT ψψφ o 2 2 )( m eE p =+′ in which the square of the angular momentum vector L is: or, in spherical coordinates: += +∇−= 22 e 22 e 2 )()()( 2 )( cmcEV m E prr ψψ h ),,(),,()( 2 ),,( 2 2 2 2 e 2 2 2 ϕθψϕθψϕθψ rrVE rm r rrr r L=−+ ∂ ∂ + ∂ ∂ h 2 2 2 2 sin 1 sin sin 1 ϕθθ θ θθ ∂ ∂ + ∂ ∂ ∂ ∂ =L We recognize here the L2 operator from our discussion on spherical harmonics. We will now consider a Hydrogen atom consisting of a single proton (Z=1) at its center and an electron surrounding it. For hydrogen-like atoms (with Z>1), the reduced mass µ =memp/(me +mp) should be used but can be neglected for this purpose of trivial illustration. 9
- 10. Assuming V=V(r), the generalized wave function for the Hydrogen Atom is: with λ as a separation constant. It is assumed that ψ =ψ (r,θ,ϕ) and its first derivatives are everywhere continuous, single-valued, and finite. The consequence of imposing these conditions are that: 2017 MRT ( )...,2,1,0)1( =+≡ lllλ The functions Y(θ,ϕ) are the spherical harmonics Yl ml(θ,ϕ) with: on which we impose the requirement (a boundary condition) that V(r)→0as r →∞. ),(),( )()()]([ 2)( 2 22 e 2 2 ϕθλϕθ λ YY rP r rPrVE m rd rPd = =−+ L h lllll ,1,...,1, ++−−≡m Substituting these into the equation above for P(r), we obtain the Radial Equation: 0)( )1( )]([ 2 22 e 2 2 = + +−+ rP r rVE m rd d ll h The equation r2[∂2/∂r2 +(2/r)∂/∂r]ψ +(2me r2/h2)(E−V )ψ =L2ψ above separates into: ),()( 1 )()()(),()(),,( ϕθϕθϕθϕθψ l ll l l lllllll m nmmn m nmn YrP r rRYrRr =ΦΘ== 10
- 11. has solutions P(r)=exp(±ar) where a=√[−(2me/h2)E]. Solutions to this equation may be obtained by first considering the behavior at large r. In the asymptotic region r →∞: 2017 MRT )(e)(e)( o2 rfrfrP arra −− == where f (r) is a function to be determined by the Radial Equation and the boundary conditions. 0)( 2)( 2 e 2 2 =+ rPE m rd rPd h If E<0, exp(+ar)→∞ as r →∞. Since this violates (i.e., the ‘mathematical’ fact that the exponential blows up at infinity!) the conditions (i.e., that it does not!) that the wave function must be finite everywhere, it is not an acceptable solution (i.e.,itis not physically possible!) On the other hand, exp(−ar)→0 as r →∞; it is therefore a possible solution. If E >0, either sign in the exponent will satisfy the boundary conditions. We concentrate on the case E<0, that is, the bound states of the atom. The asymptotic behavior suggests that solutions to the Radial Equation be sought in the form: In the following slides, we will make use of the Bohr Radius (N.B., α =e2/hc=1/137): 11 ( ) ( )MKSmorCGScm 11 2 e 2 o o 8 e 2 e 2 o 1029.5 επ4 1052.0 −− ×==×=== em a cmem a hhh α
- 12. First few radial functions Rnl(r) for R10, R20 and R21 as a function of the Bohr radius, ao = 4πεoh2/mee2 and has a value for Hydrogen of 5.29×10−11 m. 0 )1(2 επ4 1 2 2 o 2 2 = + −+− f rr e rd fd a rd fd ll The substitution of P(r) = f (r)exp(−a r) into this equation yields: To ensure that f remains finite as r→ 0, it is necessary for s to be positive. When this series is substituted into the differential equation for f , it is found that s=l +1 >0. Without immersing ourselves into the fairly intricate details, the final solution for Rnl is given by (if nuclear movement is not negligible: ao'=ao(1+ me /mN)): The Radial Equation now becomes: 0)( )1( επ4 12)( 2 2 o 2 e 2 2 = + −++ rP rr e E m rd rPd ll h To proceed further, it is necessary to specify the form of the potential V(r). Let us now assume that the physical system consists of an electronof rest mass me interactingwith a nucleus of charge e, viatheCoulomb interaction (notice how this is a function of r only): r e rV 2 oεπ4 1 )( = whose solutions may be expressed as a power series (i.e., we can solve it only approximately!): )( 2 210 L+++= rArAArf s 12 / o /2 1 1 2/3 o 2 1 o o e )e( 1 )!1()!( 2 )( 1 )( + +− −− −− + + −−+ == l l l l l l l ll ll ra r r rd d annn rP r rR anr nanr n n nn n = 1, l = 0 n = 2, l = 0 n = 2, l = 1 Rnl(r) r /ao 2017 MRT o/ 2/3 o 10 e2 1 )( ar a rR − = o2/ o 2/3 o 20 e2 2 1 )( ar a r a rR − − = o2/ o 2/3 o 21 e 32 1 )( ar a r a rR − = 12
- 13. Pnl(r) 1 0 2 0 2 1 3 0 3 1 3 2 4 0 4 1 4 2 4 3 o2 o 23 o e 2 1 2 1 arZ a rZ r a Z − − Explicit forms of Pnl(r) for several values of n and l are given in the Table below. n l o e2 23 o arZ r a Z − o2 o 223 o e 62 1 arZ a rZ a Z − o3 2 oo 23 o e 27 2 3 2 1 33 2 arZ a rZ a rZ r a Z − +− 2017 MRT o3 oo 223 o e 6 1 627 8 arZ a rZ a rZ a Z − − o3 2 o 3223 o e 3081 4 arZ a rZ a Z − o4 3 o 2 oo 23 o e 192 1 8 1 4 3 1 4 arZ a rZ a rZ a rZr a Z − − +− o4 2 ooo 223 o e 80 1 4 1 3 5 16 1 arZ a rZ a rZ a rZ a Z − +− o4 o 2 o 3223 o e 12 1 564 1 arZ a rZ a rZ a Z − − o4 3 o 4323 o e 35768 1 arZ a rZ a Z − 13
- 14. With V(r)=(1/4πεo)e2r−1 and in spherical coordinates, the Schrödinger equation reads: ϕρ ρ θ θ θ ϕθρρϕθψ l l l l l ll l ll l l l l l l l l l l l l l ll l l ll l l mi m m m nar nnar n n ar m nmnmn d d ra r r dr d am m nn YLNr e )cos( sin sin e )e( 1 )!( )!( π)!1()!( 12 !2 )1( ),()(e),,( 2 12 o 2 1 1 23 o 2 2 12 1 2 o oo + + + +− −− −− + = + −− − + − −−+ +− → ⋅= 0),,( πε4 sinπ8),,(),,( sinsin ),,( sin o 2 2 22 e 2 2 2 22 = ++ ∂ ∂ + ∂ ∂ ∂ ∂ + ∂ ∂ ∂ ∂ ϕθψ θ ϕ ϕθψ θ ϕθψ θ θ θ ϕθψ θ rE r e h rmrr r r r r )()()(),,( ϕθϕθψ ΦΘ= rRr 02 2 2 =Φ+ Φ lm d d ϕ ↓ ↓ ↓ ϕϕ ϕ ll l mimi m BA − +=Φ ee)( 0 sin )1(sin sin 1 2 2 =Θ −++ ∂ Θ∂ θθ θ θθ l ll m d d ↓ θcos= ↓ w 0 1 )1(2)1( 2 2 2 2 2 =Θ − −++ Θ − Θ − w m wd d w wd d w l ll )()( wPAw m mm l ll lll =Θ ↓ ↓ ↓ ↓ l l l l l ll l l l l l l ll l m m m m d d m m + + + + −+− =Θ )cos( sin sin )!( )!( 2 12 ! )( )( 2 12 θ θ θθ ↓ ϕ ϕ l l mi m e π2 1 )( =Φ ↓ 12 / o /2 1 1 2/3 o 2 1 o o e )e( 1 )!1()!( 2 )( + +− −− −− + + −−+ = l l l l l l l l ll ra r r rd d annn rR anr nanr n n n ↓ ↓ ↓ )(e)( 122/ ρρρ ρ + + − = l l l ll nnn LAR 0 )1( 4 22 2 = + −+++ Rn rd dR rd Rd ρ ρ ρ ll 0 )1( πε4 π81 2 o 2 2 e 2 2 2 = + + +++ R r E r e h m n rd dR r rd d r ll ↓ and the solution for the wave function becomes (which is a functionof r,θ andϕ only): 2017 MRT Time Independent Schrödinger Differential Equation Legendre Associated Differential Equation Laguerre Differential Equation Second-Order Homogeneous Differential Equation with Constant Coefficients 14 o/2 ar=↓ ρ
- 15. Notation Wave function Energy (×R) Degeneracy (n2) 1 0 0 1s −Z2 1 2 0 0 2s −(Z/4)2 4 1 0 2p0 1 ±1 2p±1 o2 o 23 o 200 e2 32π 1 arZ a rZ a Z − − =ψ To summarize, we have three quantum numbers n, l, and ml, where n is the principle quantum number with possible value 1,2,3,…; l is the orbital angular momentum quantum number (orbital quantum number, for short) with possible values 0,1,2,…, n−1; and, ml the magnetic (or projection) quantum number whose values are restricted to l, l–1,…,−l. In spectroscopic notation, s, p, d, f, g,… correspond to l=0,1,2,3,4,…, respectively. n l ml o e π 1 23 o 100 arZ a Z − =ψ θψ cose 32π 1 o2 o 23 o 210 arZ a rZ a Z − = φ θψ iarZ a rZ a Z ±− ± = esine 64π 1 o2 o 23 o 121 2017 MRT It is convenient to employ a natural unit of energy,calledaRydberg, R, for measuring the energy levels of hydrogen: 2 4 e 2h em R = 15
- 16. 2017 MRT Notation Wave function Energy (×R) Degeneracy (n2) 3 0 0 3s −(Z/9)2 9 1 0 3p0 1 ±1 3p±2 2 0 3d0 2 ±1 3d±1 2 ±2 3d±2 4 0 0 4s −(Z/16)2 16 1 0 4p0 1 ±1 3p±2 n l ml o3 2 oo 23 o 300 e21827 3π81 1 arZ a rZ a rZ a Z − +− =ψ θψ cose6 π 2 81 1 o3 2 oo 23 o 310 arZ a rZ a rZ a Z − − = ϕ θψ iarZ a rZ a rZ a Z ±− ± − = esine6 π81 1 o3 2 oo 23 o 131 )1cos3(e 6π81 1 23 2 o 23 o 320 o − = − θψ arZ a rZ a Z ϕ θθψ iarZ a rZ a Z ±− ± = ecossine π81 1 o3 2 o 23 o 132 ϕ θψ iarZ a rZ a Z 223 2 o 2/3 o 232 esine π162 1 o ±− ± = o4 3 o 2 oo 23 o 400 e24144921 π1536 1 arZ a rZ a rZ a rZ a Z − − +− =ψ θψ cose2080 π 5 2560 1 o4 3 o 2 oo 23 o 410 arZ a rZ a rZ a rZ a Z − + − = ϕ θψ iarZ a rZ a rZ a rZ a Z ±− ± + − = esine2080 2π 5 2560 1 o4 3 o 2 oo 23 o 141 16
- 17. Notation Wave function 2 0 4d0 2 ±1 4d±1 2 ±2 4d±2 3 0 4f0 3 ±1 4f±1 3 ±2 4f±2 3 ±3 4f±3 n l ml )1cos3(e20 π3062 1 24 3 o 2 o 23 o 420 o − − = − θψ arZ a rZ a rZ a Z ϕ θθψ iarZ a rZ a rZ a Z ±− ± − = ecossine12 2π 3 1536 1 o4 3 o 2 o 23 o 142 ϕ θψ iarZ a rZ a rZ a Z 224 3 o 2 o 2/3 o 242 esine12 2π 3 3072 1 o ±− ± − = ϕ θψ iarZ a rZ a Z ±− ± − = e)1cos5(e 5π 3 6144 1 24 3 o 23 o 143 o ϕ θθψ iarZ a rZ a Z 224 3 o 23 o 243 ecossine 2π 3 3072 1 o ±− ± = ϕ θψ iarZ a rZ a Z 334 3 o 2/3 o 343 esine π6144 1 o ±− ± = )cos3cos5(e 5π3072 1 34 3 o 23 o 430 o θθψ − = − arZ a rZ a Z 2017 MRT The fact that in a Hydrogen atom the energy level degeneracy (i.e., two or more different states give the same value of energy) is actually n2 is due to the special property of the Coulomb field. In fact, it is the classification of states with respect to the irreducible representations of the four-dimensional group – of which O+(3) is a subgroup – that leads to the n2 degeneracy. 17
- 18. A Hydrogen atom has an electron which is distributed around a normalized radius ao. is a complex probability density of the electron in a Hydrogen atom. ϕ θ θ θϕθψ l l l l l l ll l l l l l l l l l l l l ll l l mi m m m anr nanr n n mn d d ra r r dr d am m nn r e )cos( sin sin e )e( 1 )!( )!( π)!1()!( 12 !2 )1( ),,( 2 12 o 2 1 1 23 o 2 o o + + + +− −− −− + + − −−+ +− = Here is the generalized wavefunction of a Hydrogen atom (forjustasingleelectron): where n, ml and l are quantum numbers indicating the state ψnlml (r,θ,ϕ) of the electron’s position r given by its orbital level, magnetic moment, and angular momentum, respectively. 1 nm = 10−9 m n = 2,llll = 1 n = 2,llll = 0 n = 1,llll = 0 ψ100 ψ200 ψ211 ml=0 ml = 0 mllll = 1 mllll = 0 |ψ 84±1(r,θ,ϕ)|2 2017 MRT 18
- 19. 0 5 10 ElectronVolt(eV) 13.60 n 1 2 3 4 5 ∞ s p d f LymanSeries 0 −5 −10 −13.60 The first few energy levels of the Hydrogen atom – without fine structure (i.e. corrections due to nuclear spin angular momentum I ) or using the reduced mass µ instead of me. The energy levels of the Hydrogen atom are given by the Balmer formula: The Rydberg constant is given by: and it is related to the ‘Ry’ energy unit by: eV605.137.3169,7310 2 2 2 e2 1 == = − ∞ 1 cm c e cmR h Ry1eV(12)13.6056923 ≡ Ry −−= ∞ 2 1 2 2 2 11 nn ZREn ( )...,4,3,2,1 1 2 )( 2 2 22 22 e =−=−= n n Z n Zem En Ry h If an electron changes from one state to another, there will be a corresponding change in energy of the system: These transitions will in general be accompanied by an emission or absorption of electromagnetic radiation (c.f., Appendix: Higher Order Electromagnetic Interactions). For instance, if n1 =1, then one gets the Lyman series, in which n2 can take on values of 2, 3, 4, …. Recall also that the electron volt [eV] is a unit of energy equal to approximately 1.602×10−19 J [Joule] and by definition it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt. Thus it is 1 Volt (1 Joule per Coulomb) multiplied by the electron charge (1e, or 1.602×10−19 C [Coulomb]).Therefore,one electron volt is equal to: 1 eV =1.602×10−19 J. 2017 MRT 19
- 20. The probability of finding an electron in an element of volume dτ is: 2017 MRT If this equation is integrated over the surface of a sphere, we obtain the probability of finding an electron in a shell between two spheres of radii r and r +dr. Since the spherical harmonics are normalized to unity, the result is simply Pnl 2(r)dr. In the sense that ψ ∗ψ is a charge density, Pnl 2(r) is a radial charge density. τdYYrP r τd mm n ),(),()( 1 * *2 2 ϕθϕθψψ ll lll= The probability of finding an electron between θ and θ +dθ is proportional to: θθθθθϕθϕθ dPdYY mmm sin)](cos[sin),(),( 2* lll lll = Finally, the probability of finding an electron between ϕ and ϕ +dϕ is simply proportional to dϕ. 20
- 21. Plot forY1 0,R10,and the probability densityψ100 ∗ψ100=|ψ100|2 of the orbital [ yellow(+)/ blue (−) on black] which is the probability of finding an electron around the atom’s nucleus. 2017 MRT Plots for Y1 0 , R20, & |ψ200|2, Y1 0 , R21, & |ψ210|2, and for Y1 1 , R21, & |ψ211|2. +½ −½ +½ −½ The maximum number Nn of electrons in a shell characterized by principle number n is given by twice (i.e. due to spin) the number of orbital stateswiththatn: ...,18,8,22 )12(2 2 1 0 == += ∑ − = n N n n l l 21 + −
- 22. Plots for Y3 0 , R30, & |ψ300|2, Y0 0 , R31, & |ψ310|2, Y1 1 , R31, & |ψ311|2, Y2 0 , R32, & |ψ320|2, Y2 1 , R32, & |ψ321|2, and for Y2 2 , R32, & |ψ322|2. And finally, a few more plots for Y3 ...≤3 , R4 …≤3, & |ψ4 …≤3 …≤3|2 … 2017 MRT 22
- 23. One intricate configuration for a Hydrogen atom is for a single electron (reduced mass µ, charge e) surrounding a single proton (with proton-to-electronmass ratio mp/me ≅1836). Joule or 19- 2 32 2 2 0,2,4d4 2 22 e 2 2 2 eo 2 101.36eV85.0 4 34 4 1 4 eV6.13 24 3 ½ 1 1 2 1 2 ×=−= − +− →−≅ − + + −= ααα z n n cmn nnma E l h l 0)( επ42 420 o 2 4 2 e 2 = ++∇ r r ψ e E m h 4 222 0,2,4d42 )3( π16 52 r rzz − →ΘΦ or Schrödinger’s time-independent wave equation (i.e.,the steady state in quantum theory) in r position-vector space:* This can also be represented differently by expanding time t and the Laplacian operator ∇2 into spherical coordinates r, θ, ϕ and keeping the potential −e2/r generalized as V: ),,(),,(),,( 2 ),,( 420420 2 e 2 420 ϕθψϕθϕθψϕθψ rrVr m r t i +∇ −= ∂ ∂ h h This 4dz2 wavefunction is then for n=4, l=2 and ml=0:† † The probability distribution of the electron surrounding the protonic nucleus is – or this 4dz2 state: * The total energy required to provide the electron surrounding the protonic nucleus to reach this state is calculated as always being: )1cos3(e 1 π1536 5 )cos( sine)e(1 π04 5 32 1 24 2 o 23 o 2 42 5 42 o 62 23 o o oo − = = − θ θ θ ar arar a r ad d ra r dr rd a 4dz2 One of the many states possible for an electron to occupy in a Hydrogen atom. ||||ψ420 (r,θ,ϕ =0)||||2 2017 MRT 23
- 24. Average values of various powers of r are often needed in computation; these are defined by the expectation value: 2017 MRT Expectation values 〈rk 〉 of rk for several values of k are given in the Table below. ∫∫ ∞ + ∞ == 0 22 0 2 )()( drrRrdrrPrr n k n kk ll 〈rk〉 1 2 3 4 −1 −2 −3 −4 )]1(315[ 2 2 2 2 2 o +−+ lln n Z a k )]1(3[ 2 2o +− lln Z a )]1()1)(2(3)1)(2(30)1(35[ 8 222 2 3 2 o −+++−+−− llllllnnn n Z a ]12)1033)(1(5)322(3563[ 8 2224 4 4 4 o +−+++−+− llllllnn n Z a 2 o 1 na Z )½( 1 32 o 2 +lna Z lll )½)(1( 1 33 o 3 ++na Z )½)(½)(1)((2 )1(3 2 35 2 4 o 4 −+++ +− llll ll n n a Z 24
- 25. For the Hamiltonian H=p2/2me −(1/4πεo)Ze2/r the complete solution to the Schrödinger equation Hψ =Eψ for the bound states consists of the orbitals ψnlml (r,θ,ϕ) together with the energy eigenvalues En =−Z2/n2. However, the Schrödinger equation: 2017 MRT with the Hamiltonian (see the Appendix – Higher Order Electromagnetic Interactions): pAAp ××××∇∇∇∇ΣΣΣΣ∇∇∇∇∇∇∇∇××××∇∇∇∇ΣΣΣΣ ϕϕ •−•−−•+ += 22 e 22 e 2 23 e 4 e 2 e 48822 1 cm e cm e cm p cm e c e m H hhh ψψψϕ )()( Orbit-SpinDarwinicRelativistnInteractioo HHHHHHeE ++++==+′ contains additional terms (e.g., Unperturbed, Interaction, Relativistic, Darwin and Spin- Orbit) which all have an effect on the eigenfunctions and eigenvalues of the system. which identify the system as a particle with spin s=½. ±=±+=± ±±=± 222 ¾)1½(½ ½ hh h S zS Examination of the Hamiltonian above reveals the presence of terms containing the operator ΣΣΣΣ whose components are the Pauli matrices, σσσσ =[σ1,σ2,σ3] (where σ1 REAL/SYM = +1, σ2 COMPLEX/ANTI = mi, and σ3 REAL/DIAG PARITY =±1). According to our previous discussion, S =½hΣΣΣΣ where the rectangular components of S are angular momentum operators and: Thus, the appearance of ΣΣΣΣ in the Hamiltonian indicates that the wave function of the system must include a spin eigenfunction. 25
- 26. In the spin-½ [in units of h] case, we saw earlier that: 2017 MRT = =−=− = =+=+ = − + χ χ 1 0 ½,½ 0 1 ½,½ , sms The one-electron spin function is: −= += == − + ½ ½ )( s s sm m m ms for for χ χ ξξ whereas the (one-electron) spin orbital is: )()(),,,(),,( ssmmn mmmnr s ξrξ ψψϕθψ == ll ll It shall be understood that an integral involving spin orbitals implies a spatial integration as well as a summation over spin coordinates. Also, we now have a degeneracy of 2n2 associated with the addition of spin eigenfunctionψnlmlms (r,θ,ϕ,±½) andweuse |l,s;ml ,ms 〉 to identify the angular and spin parts of the wave function. Degenerate eigenfunctions may be combined linearly to form other sets in a coupled representation of degenerate eigenfunctions using the Clebsch-Gordan coefficients Cj mlms =〈l,s;ml,ms |l,s; j,mj 〉: ∑∑ == s s s mm s j mm mm jssj mmsmjsmmsmmsmjs l l l lll lllll ,;,,;,,;,,;,,;, C 26
- 27. n = 1= 1= 1= 1 mllll = 0= 0= 0= 0 llll = 0= 0= 0= 0 llll = 1= 1= 1= 1 llll = 2= 2= 2= 2 llll = 3= 3= 3= 3 n = 2= 2= 2= 2 mllll = 0= 0= 0= 0 n = 2= 2= 2= 2 mllll = 1= 1= 1= 1 n = 3= 3= 3= 3 mllll = 0= 0= 0= 0 n = 3= 3= 3= 3 mllll = 1= 1= 1= 1 n = 3= 3= 3= 3 mllll = 2= 2= 2= 2 n = 4= 4= 4= 4 mllll = 0= 0= 0= 0 n = 4= 4= 4= 4 mllll = 1= 1= 1= 1 ψ100 ψ200 & ψ210 ψ211 ψ300, ψ310 & ψ320 ψ311 & ψ321 ψ322 ψ400, ψ410, ψ420 & ψ430 ψ411, ψ421 & ψ431 Atomic orbitals are best represented by various probability distributions where the electron will most probably be present given the quantum numbers n and ml versus llll. LEVEL 1 – FUNDAMENTAL 1H ENERGY LEVEL LEVEL 2 – FIRST EXCITATION AVAILABLE LEVEL 2 – ADDED SECTORIAL HARMONICS LEVEL 3 – SECOND EXCITATION AVAILABLE LEVEL 3 – MORE SECTORIAL HARMONICS LEVEL 3 – ADDED TESSERAL HARMONICS 2017 MRT 27
- 28. No more than 2 electrons in this single 1s orbital. No more than 6 electrons in these 2p (px, py, & pz ) orbitals. No more than 2 electrons in this 2s orbital. No more than 6 electrons in these 3p (px, py, & pz ) orbitals. No more than 2 electrons in this 3s orbital. No more than 10 electrons in these 3d (dxz, dyz ,dxy, d x2 −−−−y2 & dz2 ) orbitals. No more than 18 electrons in these 4 f (fz3 −−−−(3/5)zr2 , fy3 −−−− (3/5)yr2 , fx3 −−−−(3/ 5)xr2 , fx(z2 −−−−y2) , fy(x2 −−−−z2) , fz(x2 −−−−y2) & fxyz) orbitals. ψ1s ψ2s & ψ2pz ψ2px ψ3s, ψ3pz & ψ3dx2 ψ3px & ψ3dxz ψ3dxy ψ4s , ψ4pz , ψ4dz2 & ψ4f z3 −−−−(3/5)zr2 ψ4px , ψ4dxz & ψ4f y(x2−−−− z2) 1s 2s 2pz 2px 3dz2 3pz 3px 3dxz 3dxy 3s 4fz3−−−−(3/5)zr2 4fy(x2−−−−z2) Each electron can have a Spin Up component(Sz =+½h) and a Spin Down (Sz =−−−−½h) along the z-axis within each orbital – both not both.This is Pauli’s Exclusion Principle. llll = 0= 0= 0= 0 llll = 1= 1= 1= 1 llll = 2= 2= 2= 2 llll = 3= 3= 3= 3 2017 MRT 28
- 29. We turn now to the spin-orbit coupling term in the Schrödinger equation. The Hamiltonian HSpin-Orbit is given by (the reduced mass µ =memp/(me +mp) is negligeable): Spin-Orbit Coupling 2017 MRT in which L (=r××××p) is the orbital angular momentum operator. When ∇∇∇∇φ ××××p=(dφ/dr)(1/r)L is substituted into HSO above and σσσσ is replaced by (2/h)S we obtain: 322 e 22 2 )( rcm eZ r h =ξ p××××∇∇∇∇ΣΣΣΣ φ•−=≡ 22 e SOOrbit-Spin 4 cm e HH h where e is the electronic charge (we also set henceforth e=e/4πεo ), me is the rest mass of the electron and φ is the electrostatic potential. If φ depends only on r, we have: r r ˆφ φ φ ′== rrd d ∇∇∇∇ and: Lprp rrrd d φφ φ ′ == h××××××××∇∇∇∇ 1 SLSL •=•−= )( 1 2 22 e 2 SO r rcm e H ξ h For Hydrogen, the potential is φ =Ze/r so that: which is the spin-orbit amplitude (or intensity). = oπε4 e e 29
- 30. The one-electron Hamiltonian with spin-orbit coupling now becomes: 2017 MRT with: SO0 HHH += SL•=−∇−= )( 2 SO 2 2 e 2 0 rH r eZ m H ξand h 0)1(SO =− jinji EH δ )0( SO )0(SO jninji HH ψψ= We regard HSO as a perturbation (i.e., an approximation based on energy or potential series) although the justification for this may not be apparent until a calculation of the magnitude of the effect has been carried out. where: and ψ (0) ni, ψ (0) nj are degenerate eigenfunctions of Ho. Since H0 has degenerate eigenvalues, the first-order corrections to the energy with be obtained from the solution of the secular (or determinant) equation: Without proof, we state that |l,s;j,mj 〉 is a simultaneous eigenfunction of L2, S2, J2, Lz, and L•S. We therefore expect the coupled representation to be the most convenient since only diagonal matrix elements will appear in the secular determinant. 30
- 31. To see how this works out, let: 2017 MRT or: )( 222 2 1 SLJ −−=•SL ∫∫ ∞∞ ==== 0 2 0 2 )()()()(,)(,)( rdrPrrdrrRrnrnr nnn lll ll ξξξξξ 3322 e 22 3322 e 22 1 2 , 1 , 2 )( rrcm eZ n r n rcm eZ r h ll h ==ξ Using the coupled representation: The Hamiltonian HSO =ξ(r)L•S also contain the radial function ξ(r). So, to obtain the energy corrections it is also necessary to evaluate the expectation value of ξ(r): From the Table for 〈rk 〉 (with k=−3): SLSLJSLJ •++== 2222 and++++ jjss jjjj ssjj mjsSLJmjsmjsmjs ′′′+−+−+= −−′′′′=•′′′′ δδδ llll llll )]1()1()1([ ,;,,;,,;,,;, 2 1 222 2 1SL In hydrogen, ξ(r) is given by ξ(r)=Ze2h2/2me 2c2r3 in which case: lll )½)(1( 11 23 o 3 3 ++ = na Z r 31
- 32. On combining the above relations for 〈l′,s′; j′,m′j|L•S|l,s;j,mj 〉, ξnl, and 〈r−3 〉, the spin- orbit interaction energy is ( j=l+½): 2017 MRT or in Rydberg units: lll llh )½)(1( )1()1()1( 4 322 e 3 o 224 SO ++ +−+−+ = n ssjj cma eZ E with α =e2/hc and ao =h2/mec2. It is possible to rewrite the above equations in terms of l since j is confined to the values l±½ and s =½. We then have (for l≠0 only): For Z=1 and l=1, the splitting due to spin-orbit coupling is 0.36, 0.12, and 0.044 cm−1 for n=2, 3, and 4, respectively. Since these energies are much smaller than the energies which separates states with different values of the principle quantum number n – about 104 cm−1 – the use of perturbation theory to first order is certainly appropriate. Ry lll ll )½)(1( )1()1()1( 3 24 SO ++ +−+−+ = ssjj n Z E α +−++ == ± )1()½)(1( 1 2 2 1 2 1 33 o 22 e 224 ½SO llll h l nacm eZ EE 1 cm− + =∆ )1( 84.5 3 4 SO lln Z E 32
- 33. From the relation 〈l′,s′; j′,m′j|L•S|l,s;j,mj 〉=½[ j( j+1)−l(l+1)−s(s+1)]δl′lδs′sδj′j it is seen that the matrix elements vanish unless: These are the selection rules for spin-orbit coupling. Also, these are also valid: 0)(0 =+∆=∆=∆=∆ sj mmmj ll and The Table below lists values of the 〈1,½; m′l,m′s|L•S|1,½;ml,ms 〉 matrix elements for p states of ξnl= 〈n,l|ξ(r)|n,l〉 (and shortened to |ml ,ms 〉 since l=1 and s =½ for all states). ½,1− 2017 MRT ½,1 ½,1 ½,0 ½,0 − ½,1− ½,1−− ½,1 − ½,0 ½,0 − ½,1− ½,1 −− 2 1 2 1 − 2 1 2 1 0 0 2 1 2 1 2 1 − 2 1 1,01,0 ±=∆±=∆ smm andl 33
- 34. The term in the Schrödinger equation that corresponds to the relativistic correction to the kinetic energy is: 2017 MRT or, with H0 =p2/2me −Ze2/r: ′+ ′+′+ +′−=′ jj jjjj jjjj mjsn r mjsneZ mjsnH r mjsnmjsn r HmjsneZ mjsnHmjsn cm mjsnHmjsn ,;,, 1 ,;,,)( ,;,, 1 ,;,,,;,, 1 ,;,, ,;,,,;,, 2 1 ,;,,,;,, 2 22 00 2 2 02 e R ll llll llll Other Interactions We shall be interested in the effects produced by HR within a manifold of states specified byparticularvaluesof n, l, s, j as for example 2S1/2 or 2P3/2 (where 2s+1Xj with X=S,P,D,F,… stands for l=0,1,2,3,…) eigenstates of H0 belonging to a particular value of n. Therefore, treating HR as a perturbation, the basis set is |n,l,s; j,mj〉 and the matrix elements are: 2 e 2 2 e 23 e 4 RicRelativist 22 1 8 −=−=≡ m p cmcm p HH + ++−= +−= 2 22 00 22 02 e 2 2 02 e R 1 )( 11 2 1 2 1 r eZH rr HeZH cmr eZ H cm H 34
- 35. This last equation for 〈n,l,s; j,m′j|HR |n,l,s; j,mj〉 can be simplified. For the first term on the right we have: 2017 MRT where En (0) is an eigenvalue of H0. In view of the non-commutativity of r and p, H0 and 1/r do not commute; nevertheless, the Hermitian property of H0 leads to the equality of the matrix elements: jj mmnjj EmjsnHmjsn ′=′ δ2)0(2 0 )(,;,,,;,, ll in which 〈r−1 〉 depends only on the radial part of the wave function and is independent of mj. Similarly: The net result is that HR has only diagonal matrix elements: 22 e 22 (0) 2 e 2 2 e 2(0) R 1 2 )(1 2 )( rcm eZ r E cm eZ cm E H n n −−−= jj mmnjjn jjjj r Emjsn r mjsnE mjsnH r mjsnmjsn r Hmjsn ′=′= ′=′ δ 1 ,;,, 1 ,;,, ,;,, 1 ,;,,,;,, 1 ,;,, )0()0( 00 ll llll jjmmjj r mjsn r mjsn ′=′ δ22 1 ,;,, 1 ,;,, ll 35
- 36. For hydrogen, we have: Ry2 2 2 4 e 2 2 o 2 2 2 )0( 22 n Z em n Z a e n Z En −= −=−= h + +−= + +−= )½( 1 4 3 )½( 11 4 1 2 22)0( 22 o 2 e )0(22 R ll nn ZE nnnacm EeZ H n n α Also, from the Table for 〈rk 〉 (with k=−1 and k=−2): Therefore: This expression holds for all values of l including l=0. )½( 1111 32 o 2 22 o + == lna Z rna Z r and with α2 =(e2/hc)2 =e2/mec2ao and ao =h2/mec2. In Rydberg units: Ry + +−−= ½ 1 4 32 3 4 R lnn Z E α 36 2017 MRT
- 37. Next we consider the Darwin term in the Schrödinger equation: 2017 MRT which is of the same order as HR. With E=−∇∇∇∇φ and φ =Ze/r: where we have used the relationship ∇2(1/r)=−4πδ (r). Since only the radial part of the wave function will influence the matrix elements of HD: and: Therefore: π 1 3 o 2 00 = an Z nψ E•≡•−=≡ ∇∇∇∇∇∇∇∇∇∇∇∇ 22 e 2 22 e 2 DDarwin 88 cm e cm e HH hh φ )( 8 π41 8 22 e 22 2 22 e 2 D r cm eZ rcm e H δ hh = ∇−= 2 100,)(, ψδ =ll nrn D 2 10022 e 22 D 2 π E cm eZ H == ψ h Thus, the matrix elements of HD are non-zero only for s states. In hydrogen: Ry3 24 3 o 22 e 3 224 D 2 n Z acmn eZ E α == h ( )only0=l 37
- 38. It is now possible to combine the expressions for ESO, ER and ED intoasingle expression which depends on n and j but not on l (or ml): 2017 MRT with j=l+½. When l=0, ESO=0, and: Ry − + −=++ njn Z EEE 4 3 ½ 1 3 24 DRSO α When l≠0, ED =0 and: Therefore, the combined effects of the spin-orbit coupling, the relativistic energy correc- tion, and the Darwin term are all included in ESO+ER +ED above. It is of interest to note that on the basis of the expression for ESO +ER +ED the energies of 2S1/2 and 2P1/2 are identical for a given n. The same result is obtained from the exact solution of the Dirac equation for hydrogen. Experimentally,a smalldifference (0.035 cm−1 or 1048.95 MHz for Z=1) between the energies of 2S1/2 and 2P1/2 has been observed. This is known as the Lamb shift and its explanation is based on higher-order radiative corrections (e.g., using Quantum Electrodynamics and considering electron self-energy, vertex correction, electron mass counter-terms, photon self-energy, &c. giving actually 1057.36 MHz). Ry −−=+ nn Z EE 4 3 13 24 DR α Ry − + −=+ njn Z EE 4 3 ½ 1 3 24 RSO α 38
- 39. A partial energy level Figure of Hydrogen is shown below for the energy levels of the Hydrogen atom for n=1, 2, and 3. The energies are listed in reciprocal centimeters (cm−1) (note that this Figure is not to scale). 2017 MRT The splitting that arises within a manifold of states belonging to the same value of n is known as fine structure. Due to the Lamb shift, the 2S1/2 level of the Hydrogen atom lies 0.035 cm−1 above the 2P1/2 level: an external electric field induces Stark splitting, as a result of which the 2S1/2-2P1/2 transition becomes possible. n 2S1/2 2P1/2 2P3/2 2D3/2 2D5/2 3 2 1 97492.208 82258.942 0 82258.907 97492.198 82259.272 97492.306 97492.342 } 0.035 cm−−−−1 39
- 40. First, it is assumed that the electron (or Hydrogen atom) is placed in a constant magnetic field B with vector potential: 2017 MRT Magnetic & Electric Fields rBA ×××× 2 1 = When A has the form A=½B××××r, ∇∇∇∇•A is identically zero and as a result of which we get p•A=A••••p. We shall confine our attention, initially, to the effects which are linear in B= ∇∇∇∇××××A; hence, the Hamiltonian describing the interaction – HI for short – with the field is: But A••••p=½(B××××r)•p=½B•(r××××p)=½B•L in which L is the orbital angular momentum operator. With the replacement of ΣΣΣΣ by (2/h)S and substitution of A••••p into HI M we have: The positive constant µB is known as the Bohr magneton: BpArΒrΒpA •+•≅ •+ +•= ΣΣΣΣ××××××××∇∇∇∇ΣΣΣΣ×××× cm e cm e cm e cm e cm e H eee 2 2 e 2 e M I 22 1 22 1 2 hh Referring to the Schrödinger equation again, the interaction terms that depend on the vector potential are: AApAApp ××××∇∇∇∇ΣΣΣΣ •++•+•+=+= cm e cm e cm e m HHH e 2 2 e 2 e 2 e nInteractioo 22 )( 22 1 h )2()2( 2 e M I SLBSLB ++++++++ •=•= B cm e H µ h J/T24 e 1027.9 2 − ×== cm e B h µ 40
- 41. The equation HI M =µB B•(L++++2S) may also be written as: 2017 MRT BµBµ SL •−•−=M IH The resemblance of HI M =−µµµµL •B−µµµµS •B to the classical expression for the energy of a magnetic dipole in a magnetic field suggests that µµµµL and µµµµS be interpreted as magnetic moment operator associated with L and S, respectively. The minus signs in −µB L and −2µB S are due to the negative charge on the electron. Note that the factor of 2 appears in the relation between µµµµS and S and is absent in the relation between µµµµL and L. The latter has a classical analog but the former does not. Actually, the factor of 2 is slightly erroneous; higher order corrections shows that: although in most cases it is sufficient to set ge =2. where: SµLµ SL BB µµ 2−=−= and SµS Bµge−= with: 0023.2e =g 41
- 42. It is important to distinguish between a magnetic moment operator µµµµ such as µµµµL and µµµµS defined by µµµµL=−µB L and µµµµS=−2µB S from the quantity µ known as the magnetic moment. The orbital magnetic moment µL is defined as: 2017 MRT llll ll === mm L z ,, µµL where µz L is the z-component of µµµµL. From µµµµL=−µB L: In other words, the absolute value of the spin magnetic moment of the electron is one Bohr magneton. So, in place of the relations µµµµL=−µB L and µµµµS=−2µB S we may now write: lllll ll BzB mLm µµµ −===−= ,,L Similarly, the spin magnetic moment µS is given by: BB BszsBs S zs g sgsmsSsmssmssms µµ µµµµ −≈−= −===−==== e e 2 1 ,,,,S SSµLµ S S S L L µ s µµ 2=== and l It is convenient, although not essential, to assume that the coordinate system has been chosen so that the z-axis coincides with the direction of B. In this case, the relation HI M =µB B•(L+2S) simplifies to: )2(M I zzB SLBH += µ where B=Bz. 42
- 43. We shall now divide the discussion of magnetic field effects into two parts: ‘weak’ fields and ‘strong’ fields. The scale is set by the spin-orbit interaction energy. If the changes in energy due to the application of a magnetic field are small compared with the spin-orbit coupling energy, the field is said to be ‘weak’; otherwise it is strong. The ‘weak’ field case is the regime of the ordinary Zeeman effect while the ‘strong’ field case corresponds to the Paschen-Back effect. 2017 MRT When the fields are ‘weak’ it is presumed that the effects of spin-orbit coupling have already been taken into account so that the eigenstates are described in the coupled representation |l,s; j,mj 〉. We shall therefore be interested in matrix elements of HI M in this basis set. To evaluate such matrix elements, we apply the Landé formula (which is a special form of the Wigner-Eckart Theorem and stems from irreducible tensors T(k) with k=1 which we have not discussed): But: and we have used (L ++++2S)•J=(J ++++S)•J=J2 ++++S•J. Now, since L=J−−−−S, L2 =J2 ++++S2 −2S•J and S•J=½(J2 +S2 −L2) we have: jj mmjjzj mmjsJmjs ′=′ δ,;,,;, ll jzj jj jzzj mjsJmjs jj mjsmjs mjsSLmjs ,;,,;, )1( ,;,)2(,;, ,;,2,;, ll ll ll ′ + • =+′ JSL ++++ )( 2 1 2 3 )2( 222 LSJ −+=•JSL ++++ 43
- 44. Therefore: 2017 MRT Substituting these key results into the Landé formula we obtain: which indicates that only diagonal elements are non-zero. Hence the energies in a “weak” magnetic field are given by: jj mmjJjzzj mgmjsSLmjs ′=+′ δ,;,2,;, ll factorLandé gg jj ssjj ssjj mjsLSJmjsmjsjmjs J jjj =≡ + +−+++ += +−+++= −+=• )1(2 )1()1()1( 1 )1( 2 1 )1( 2 1 )1( 2 3 ,;,)(,;,,;,)2(,;, 22 2 12 2 3 ll ll llll JSL ++++ These are known as the Zeeman levels with energies proportional to the magnetic number mj. Thus, the effect of the magnetic field has been to remove the mj-degeneracy. The Landé g factor may also be written as: jJB mBgE µ=M I )1(2 )1()1()1( )1(1 e + +−+++ −+= jj ssjj ggJ ll to permit the use of the more exact value of ge given by ge =2.0023. 44
- 45. It is now possible to define a total magnetic moment operator µµµµJ by: 2017 MRT Hence µµµµJ=−µB gJ J above may written as: and, in terms of µµµµJ, the magnetic Hamiltonian HI M =µB B•(L++++2S) is: JµJ JB gµ−= which contains µµµµL=−µB L and µµµµS=−2µB S as special cases. Along the train of development leading the matrix elements for µL and µS we have, for the total magnetic moment: jgjmjJjmjgjmjjmj JBjzjBJj J zj µµµµ −===−==== ,,,,J Jµ J J j µ = BµJ •−=M IH which then leads directly to the relation for EI M =µBgJBmj. Also,on comparing HI M =−µµµµJ •B above to HI M =−µµµµL •B−µµµµS •B, it is seen that: SLJ µµµ ++++= 45
- 46. For an electron in an s state (2S1/2), l=0, s=½, j =1/2, gJ =2 so that the energies from EI M =µB gJ Bmj are: as shown in the Figure below. 2017 MRT BE Bµ±=M I as shown in the Figure below. BBE BE BB B µµ µ 3 2 2/3 2M I 3 1 2/1 2M I 2)P( )P( ±±= ±= and The energy separations in 2P1/2 and 2P3/2 are: 2P1/2 mj EI M (1/3)µBB −(1/3)µBB (1/3)µBB +1/2 −1/2 gJ=2/3 2P3/2 mj EI M (2/3)µBB −(2/3)µBB +1/2 −1/2 gJ=4/3 2µBB+3/2 −2µBB−3/2 WEAK FIELD WEAK FIELD 2S1/2 mj EI M µBB −µBB 2µBB +1/2 −1/2 gJ = 2 WEAK FIELD 46
- 47. As the strength of the field is increased to the point where the splitting is comparable to the spin-orbit coupling, it is no longer legitimate to isolate a single term with a specific value of j. 2017 MRT )2()(M I zzB SLBrH ++•=′ µξ SL We forgo the development (c.f., M. Weissbluth) and enunciate only the result: 2 1 2 4 9 2 1 2 1 4 9 2 1 2 1 2 1 2 6 2/1 2 5,4 2/1 2 3,2 1 +−= + − ± +−= + + ± −= += ll llll llll ll n B n n B n B n B n n B n B n B n n B n BE BBBE BBBE BE ξ µ ξ ξ µ ξ µ ξ µ ξ ξ µ ξ µ ξ µ ξ ξ µ ξ In place of HI M =µB B•(L++++2S), the Hamiltonian must now include both the spin-orbit interaction and the magnetic field terms: 47
- 48. +5 +4 +3 +2 +1 0 −1 −2 −3 −4 −5 E/ξnl +1 +2 +3 ml=1, ms=−½ ml=−1, ms=½ ml=1, ms=½ 2P1/2 2P3/2 µBB/ξnl 2017 MRT µBB >> ξnl: This is the Paschen-Back region. In this approximation the energies conform to the expression: )2(M I sB mmBE += lµ µBB << ξnl: If we confine ourselves to linear terms in µBB, the reduction of the {E1, E2,3, E4,5, E6}/ξnl gives: .,, ,,, BEBEBE BEBEBE BnBnBn BnBnBn µξµξµξ µξµξµξ 2 2 2 1 63 1 53 2 2 1 4 3 1 33 2 2 1 22 1 1 −=−−=−= +−=+=+= lll lll These energies are plotted in the Figure below for a few special case of interest such as the transition from weak to strong magnetic field for a 2P term. >>1 ln B B ξ µ ml=−1, ms=−½ ml=0, ms=−½ ml=0, ms=½ Paschen-Backregion 48
- 49. The correlation between the “weak” field and “strong” field levels are shown in the Figure below. Note that states with the same value of mj (=ml +ms) do not cross. A further point to note is that, when an atom is subjected to a magnetic field, the Hamil- tonian is no longer invariant under all three-dimensionalrotationsbut only under rotations about an axis parallel to the magnetic field. In other words, the symmetry has been re- duced from O+(3) to a group called C∞. The consequence of this restriction in symme- try is that the Hamiltonian no longer commutes with J2, although it commutes with Jz. p ms EI M = µBB(ml + ms) µBB 0 +½ −½ 2µBB+½ +½ SPIN-ORBIT SPLITTING ml +½ 1 1 −1 −µBB−½ −2µBB−½ 0 −1 WEAK FIELD STRONG FIELD ξnl ½ξnl 2P1/2 2P3/2 mj = 3/2 mj = 1/2 mj = −1/2 mj = −3/2 mj = 1/2 mj = −1/2 BE Bn µξ 22 1 1 += l BE Bn µξ 3 2 2 1 2 += l BE Bn µξ 3 1 3 +−= l BE Bn µξ 3 2 2 1 4 −= l BE Bn µξ 3 1 5 −−= l BE Bn µξ 22 1 6 −= l J L S B L S B 2017 MRT 49
- 50. Electric fields may also have an effect on the states of an atom. This is known as the Stark effect. If the coordinate system is chosen so that the z-axis coincides with the direction of the electric field, the Hamiltonian for the interaction is: θcosE I rEezEeH == The situation of greatest physical interest is the one in which the splittings due to the Stark effect are large compared to the spin-orbit splittings. Matrix elements for the Stark effect in Hydrogen with n=2, s =½, ms = m′s. 1 cm− = 3.1 3 o Z aEe In hydrogen, assuming E =104 V cm−1 and Z =1:ZaEe o3 0 |0,0〉 |1,0〉 |1,1〉 |1,−1〉 which is considerably larger that the fine structure splitting. It is seen that because of the degeneracy of states with different l and the same n there is a linear Stark effect in hydrogen. At very high field strengths a quadratic effect appears, superimposed upon the linear effect, and results in an asymmetric shift of energy levels. 〈0,0| 〈1,0| 〈1,1| 〈1,−1| 0 ZaEe o3 2017 MRT The Hamiltonian matrix is the shown in the Table below with eigenvalues: Z aEe Z aEe E ooStark I 3 00 3 −= and,, The two states with ml =0 are shifted up and down symmetrically while the states with ml =±1 are not affected by the electric field. Thus, the ml degeneracy is only partially lifted. 50
- 51. The most important interaction between a nucleus of charge Ze and an electron of charge e is, of course, the Coulomb interaction −Ze2/r. All other interactions between a nucleus and the electrons of the same atom are classified as hyperfine interactions, and among these the most important are the ones that arise as a result of a nucleus possessing a magnetic dipole moment (i.e., associated with the nuclear spin) and an electric quadrupole moment (i.e., associated with a departure from a spherical charge distribution in the nucleus). In the former case the nuclear magnetic dipole moment interacts with the electronic magnetic dipole moments which are associated with the electronic orbital and/or spin angular momenta. In the latter, the interaction occurs when, at the position of the nucleus, the electronic charge distribution produced an electric field gradient with which the nuclear electric quadrupole moment can interact. Magnetic field created by the proton. Outside the proton, the field B is that of a dipole – µµµµI; inside, the field Bi depends on the exact partition of the magnetism of the proton. 5 22 o 5 o 5 o 3 π4 µ 3 π4 µ 3 π4 µˆˆˆ r rz B r zy B r zx BBBB zyxzyx − ===⇔++= IIIkjiB µµµ and, The magnetic field inside the proton, Bi, is given by (SI system): Consider a proton of radius ro. The partition of magnetism inside the proton creates a field B outside which can be calculated by attributing to the proton a magnetic moment µµµµI (which is taken to be parallel to the z-axis – see Figure). Hyperfine Interactions z y x B µµµµI Bi 3 o o 2 π4 µ r Bi Iµ= 2017 MRT where µo is the magnetic permeability of free space. ro The external field is thus purely dipolar. For r >> ro, we obtain the components of B (by calculating the rotational of AI = (µo/4π)[(µµµµI ×××× r)/r3]) (SI system): 51
- 52. To derive the form of this interaction we return to the Dirac equation(mo ≡me and q≡−e): with: 2017 MRT It is advantageous to separate the Hamiltonian into two parts: where: u e u )()( 2 1 )( ψψφ ππ ••=+′ ΣΣΣΣΣΣΣΣ K m eE φecmE cm KcmEE ++′ =−=′ 2 e 2 e2 e 2 2 and HFo e2 1 HHe c e K c e m H +=− +• +•= φApAp ΣΣΣΣΣΣΣΣ φeKH −••= )()(o pp ΣΣΣΣΣΣΣΣ HHF is the hyperfine Hamiltonian and it is the quantity we will be deriving. u e2 1 ψφψ − +• +•=′ e c e K c e m E ApAp ΣΣΣΣΣΣΣΣ which becomes (with ππππ=p++++(e/c)A): 52 and: )]()[( 2 )]()()()[( 2 2 e 2 e HF AApAAp ••+••+••= ΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣ K cm e KK cm e H
- 53. We concentrate on HHF which contains the entire dependence on the vector potential A and the last term on the right is quadratic in A; it may therefore be neglected in a first approximation. 2017 MRT Also, since: we have: and HHF becomes: )()(])()([)(()( r r rprrrrp f rrd dK ifKKffKifKifΚ hhh −=+−=−= ∇∇∇∇∇∇∇∇∇)∇)∇)∇) rrd dK K r =∇∇∇∇ rdr dK iKΚ r pp h−= ••+••−••= ))(())(( 1 ))(( 2 e HF pAArAp ΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣΣ K rdr dK iK cm e H h The potential φ is assumed to be a function of r only (i.e., φ(r)) which means that K depends only on r (i.e., K(r)) so that: Using the identity (ΣΣΣΣ•A)(ΣΣΣΣ•B)=A•B+iΣΣΣΣ•(A××××B), HHF is converted to: )]([ 1 )()( 2 e HF ArArpAAppAAp ××××ΣΣΣΣ××××××××ΣΣΣΣ •+•−+•+•+•= i rdr dK iKiK cm e H h 53
- 54. It is now necessary to specify the vector potential A. 2017 MRT 23 ˆ rr rµrµ A ×××××××× ΙΙΙΙΙΙΙΙ == Using the vector identities ∇∇∇∇•(a××××b)=b•(∇∇∇∇××××a)−−−−a•(∇∇∇∇××××b) and ∇∇∇∇××××ka)=∇∇∇∇k××××a−−−−k∇∇∇∇××××a, and assuming that µµµµI=0 outside of the origin so that ∇∇∇∇××××µµµµI, it is found that ∇∇∇∇•A=0 and r •A= 0. We also have p•A=ih∇∇∇∇•A=A•p and p××××A=−ih∇∇∇∇××××A−−−−A××××p. The result is: •+•+•= )( 1 )(2 2 e HF ArApA ××××ΣΣΣΣ××××∇∇∇∇ΣΣΣΣ rrd Kd KK cm e H hh The nucleus is presumed to be a point dipole with a magnetic dipole moment µµµµI; hence the vector potential at r is: •+ •+ •= 2 I 2 I 3 e HF ˆ1ˆ 2 2 rrrd Kd r K r K cm e H rµ r rµ Lµ ×××× ××××ΣΣΣΣ ×××× ××××∇∇∇∇ΣΣΣΣΙΙΙΙ hh h and with A=µµµµI ××××r/r2: This expression may be put into another form by using A=(µµµµI××××r)/r3 so that: Lµprµp rµ pA •=•=•=• ΙΙΙΙΙΙΙΙ ΙΙΙΙ ×××× ×××× 333 )( 1 rrr h 54
- 55. 2017 MRT But: Therefore: )(π4 1 1111 3 rµµ µ A rµ µµµµ µ δΙΙΙΙ 2222 ΙΙΙΙ ΙΙΙΙ2222 ΙΙΙΙ ΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙ ΙΙΙΙ ×××× ×××× ∇∇∇∇××××××××∇∇∇∇××××∇∇∇∇++++××××∇∇∇∇××××∇∇∇∇ −= ∇= ∇ == −= = = rr rrrrrr ΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙ ++++++++++++++++∇∇∇∇ µkjikjirµ == ∂ ∂ + ∂ ∂ + ∂ ∂ =• ˆˆˆ)ˆˆˆ()( zyxzyx µµµzyx z µ y µ x µ 35 3 )( rrr ΙΙΙΙ ΙΙΙΙ ΙΙΙΙ ∇∇∇∇∇∇∇∇ µr rµ µ −•= • Since µµµµI is constant, the vector product terms break down as: rµ r rµrµrµ rµµ rµ µµµ µ )( 13 )()( 11 )( 111 35333 3 ∇∇∇∇∇∇∇∇++++∇∇∇∇∇∇∇∇∇∇∇∇∇∇∇∇ ∇∇∇∇∇∇∇∇∇∇∇∇∇∇∇∇ ΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙΙ ΙΙΙΙΙΙΙΙ ΙΙΙΙ ΙΙΙΙΙΙΙΙΙΙΙΙ ΙΙΙΙ •−•= • •−= • •−= • • −= •=•+ •= • rrrrrr rrrrr in first order of the Gradient, ∇∇∇∇, and then to second order via the Laplacian, ∇2. And the same for the scalar product terms: 55
- 56. With these relations, we get: Finally: 2017 MRT When these relations are included into our last development for HHF one obtains: in which: cm e B e22 hh == µandΣΣΣΣS )(π4 3 )( 35 2 rµ µr rµ µµµ A δΙΙΙΙ ΙΙΙΙ ΙΙΙΙ ΙΙΙΙΙΙΙΙΙΙΙΙ ++++−−−−∇∇∇∇∇∇∇∇××××∇∇∇∇××××∇∇∇∇××××∇∇∇∇ rrrrr •= ∇− •= = 333 )( ])[( 1 ][ 1 rrrr rµrµ rµrµrrrµrAr ΙΙΙΙΙΙΙΙ ΙΙΙΙΙΙΙΙΙΙΙΙ −−−−))))))))((((−−−−))))××××((((×××××××× • =••== •• + • + •+ •• + • = 4253HF ))(( 2)(π4 ))((3)( 2 rrrd dK rr KH BB rSrµSµ Sµr rSrµSLµ II I II µδµ −−−− with ΣΣΣΣ whose componentsare Pauli matricesσσσσ=[σ1,σ2,σ3]. We now examine K and dK/dr. rZecmE cm K 22 e 2 e 2 2 ++′ = If ϕ is replaced by Ze/r, we have: 56
- 57. It will now be assumed that E–mec2 <<2mec2, but that Ze2/r and 2mec2 may be comparable in magnitude. Hence: When Z=1, ro =1.4×10−13 cm; this is the nuclear dimension and is much smaller than the Bohr radius which is ao =0.52×10−8 cm (about 30000 times smaller in fact!) Thus: 2 2 2 1 2 e 2 2 e 2 o o 137 1 2 1 2 1 2 1 2 1 == = = − α c e emcm e a r h h )(1 1 )1)(2(1 1 o 2 e 2 rrrcmZe K + = + = Variation of K and dK/dr with r. K(r) rises from zero at r=0 to almost unity in a distance of seve- ral units of ro, i.e., in a distance very small compared to ao (see Figure). K(r) may therefore be approximated by a step function: ( ) ( )00)(00)( ss 2 100ss ≠==== rrKK atandat ψδψψψδψ rr because ψl≠0 =0 at r=0, and δ(r)= 0 at r≠0. Assume first, that we are dealing with an s state. Then, since K= 0 at r =0 and δ(r)=0 at r≠0: With the approximation for K it may now be shown that the δ - function term does not contribute. 0 1 2 3 4 5 6 7 8 9 10 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 2017 MRT K dK/dr r/ro ≠ = = 01 00 )( r r rK when when For a state with l≠ 0: ≠ = =≠≠ 0 0 0)( 00 r r atll ψδψ r 57
- 58. Therefore, our last expression for HHF may now be written without the δ function term: where: 2017 MRT ba HHH HFHFHF += •• + • = •• + • = 42HF 53HF ))(( 2 ))((3)( 2 rrrd dK H rr H B b B a rSrµSµ rSrµSLµ II II µ µ −−−− and where the factor K that multiplies HHF a has been set to unity. 58
- 59. Let us now consider HHF b and let: and, with T=B, J=S, j=s, we obtain: 2017 MRT with: and: 2 4 1 ))(( 4 1 ))(( r=••=•• rσrσrSrS jj jj jj mjmj jj mjmj mjmj ′ + • =′ ,, )1( ,, ,, J JT T Applying the Landé formula: • −−= 42 )( 2 rrdr dK B rSrS B µ BµI •−=b HHF with: ss ss ss msms ss msms msms ′ + • =′ ,, )1( ,, ,, S SB B ssBss ms rrdr dK msmsms , )( ,2,, 4 2 2 2 • −−=• rSS SB µ 59
- 60. Therefore: 2017 MRT where 〈(dK/dr)(1/r2)〉 is an integral taken over the radial part of the wave function. But, it is possible to express dK/dr by K′=4πr2δ(r): or: Substituting this into the Landé formula above gives: so that HHF b becomes: )(π4 1 2 rδ= rdr dK )(π4 rSB δµB−=• ( )½ 1 4 1 )1( 1 2, 4 1 ,2 ,, 2 222 2 =−= −+−= −−= •≡• s rdr dK ss rdr dK ms rrdr dK ms msms B BssB ss forµ µµ S SBSB SrBSB )( 3 π16 ,, 3 π16 ,, δµµ BssBss msmsmsms −=⇒′−=′ SµrBµ II •=•−= )( 3 π16 HF δµB b H 60
- 61. Collecting terms, the magnetic hyperfine Hamiltonian acquires the form: The nuclear magnetic moment operator µµµµI is related to the nuclear spin operator I by an expression of the same form that relates electronic magnetic moments to their respective angular momenta (i.e., from the Landé factor gJ): where: is the nuclear Bohr magneton. Here mN is the mass of the proton (i.e., the nuclear mass) and gN a factor whose magnitude and sign are characteristic of each nucleus(see Table). J/T27 N N 100505.5 2 − ×== cm eh µ •+ •• + • = Sµr rSrµSLµ I II )( 3 π8))((3)( 2 53HF δµ rr H B −−−− IµI NNµg= I gN µI γ Q I gN µI γ Q (×104 rad/SG) (×10−24 cm2) (×104 rad/SG) (×10−24 cm2) 1H ½ 5.586 2.793 2.675 14N 1 0.404 0.404 0.193 7.1×10−2 2H 1 0.857 0.857 0.411 2.77×10−3 15N ½ −0.566 −0.283 0.271 n ½ −3.826 −1.913 1.832 16O 0 12C 0 17O 5/2 −0.757 −1.893 0.363 −4×10−3 13C ½ 1.404 0.702 0.673 19F ½ 5.254 2.627 2.516 35Cl 3/2 0.548 0.821 0.262 −7.97×10−2 2017 MRT 61
- 62. It is also customary to write the relation µµµµI =gN µNI as: where γ =gNµN /h is known as the gyromagnetic ratio. Following the discussion we had earlier for magnetic fields, the magnetic moment of the nucleus µI is defined as: 2017 MRT in which the coefficient in front of the square brackets can be put in several equivalent forms: The expression for HHF is the most common expression for the magnetic hyperfine interaction. The first two terms in the bracket taken together are known as the dipole- dipole interaction in analogy with the corresponding classical expression. The last term is the Fermi contact interaction; it has no classical analog. It’s totally ‘quantum’! NNeeNN22 µµγµµµγµ gggg BBBB =≈= hh IµI hγ= IIgImIIImIgImIImI IzII I zI hγµµµµ ======== NNNN ,,,,I which permits the magnetic moment operator to be written as: I I µ I I µ = Substituting the relation µµµµI =γ hI into our expression for HHF, we obtain: •+ •• + • = SIr rSrISLI )( 3 π8))((3)( 2 53HF δγµ rr H B −−−− h 62
- 63. An alternative expression for the magnetic hyperfine Hamiltonian is obtained by transforming the dipole-dipole part by means of the Landé formula. Let Then: 2017 MRT and: But since (L –S)•J=(L –S)•(L+S)=L2 −S2 and recalling that | j,mj〉=|l,s; j,mj 〉 is an eigenfunction of L2 and S2 (as well as J2 and Jz ), we get 〈 j;mj|(L–S)•J| j;mj〉= 〈 j,mj|L2 −S2 | j,mj〉=l(l+1)−s(s+1). Also r•J=r•(L+S)=r•(r××××p)/h+r•S=r•S=S•r. Therefore, as we obtained earlier (r•J)(r•S)=(r•S)(r•S)=(1/4)(ΣΣΣΣ•r)(ΣΣΣΣ•r)=r2/4. Integrating over the radial part of the wave function we obtain 〈 j,mj |B′•J| j,mj〉= −2µB[l(l+1)−s(s+1)+¾]〈1/r3〉=−2µBl(l+1)〈1/r3〉 (for s=½). so that: • +−=′ 53 )(3 2 rr B rSrSL B −−−− µ ( )½ 1 )1( )1( 2 3 = + + −=′ s rjj B for ll µB sjj jj jj mjmj jj mjmj mjmj ′ + •′ =′′ ,, )1( ,, ,, J JB B jjBjj mj rr mjmjmj , ))((3 ,2,, 53 rSJrSL SB •• +−=•′ −−−− µ 63
- 64. We may now replace B′ and substitute it in HHF. The Hamiltonian, for s=½, then take the form: where, for Hydrogen ψ 100 =(1/√π)ao 3/2, and the hyperfine coupling constant AF is given by: 2017 MRT The first (dipole) term in HHF is zero when l=0; on the other hand, the second (contact) term is zero when l≠0, which means, then, that the two parts of the magnetic hyperfine interaction do not overlap. For s states, we need only consider the contact interaction, whereas for non-s states, only the dipole part contributes. )( 3 π16 2 100 2 100 rδψψγµ == andhBFA JIJI SIJI •+• + + = •+• + + = FB B A rjj rjj H 3 2 1003HF 1 )1( )1( 2 3 π81 )1( )1( 2 ll h ll h γµ ψγµ As in the case of spin-orbit coupling, the latest form of HHF suggest that it would be useful to couple the angular momenta I and J to form a new angular momentum operator: with the notation |j;mj〉, |I;mI〉 and |F;mF〉 to designate the eigenfunctions belonging to J, I and F, respectively. SLIJIF ++=+≡ 64
- 65. For Hydrogen in an s state only the contact term is effective. Since l=0, J may be replaced by S so that: and, since the spin of the proton is I=½, we have also s=½ thus F=0 & 1. Hence: which indicates that the only diagonal elements are non-zero. For the two possible values of F, the energies are: ( ) ( ) =−=− == = 0 4 3 π4 1 4 1 3 π4 2 100 2 100 FA FA E FB FB ψγµ ψγµ h h )( 2 1 222 ISFSI −−=• −+= +−+−+=−−=• 2 3 )1( 2 1 )]1()1()1([ 2 1 ,)(½,,, 222 FF IIssFFmFmFmFmF FFFF ISFSI 2S1/2 0.047 cm−1 (or 21 cm) F = 1 F = 0 n = 1 Magnetic hyperfine splitting of the ground state of hydrogen. The entire splitting is due to the Fermi contact term geµBgNµN (i.e., a quantum effect.). o 2 100 3 61 3 6π1 a AE B FB h h γµ ψγµ ===∆ For hydrogen, |ψ 100|2 =1/πao 3 (ao =0.529×10−8 cm), γh =µN gN = 5.05×10−23 erg/GHz and with µB =0.927×10−20 erg/GHz, the difference in energy between the two levels for E is: 2017 MRT as show in the Figure. If we set ∆E= hν, the frequency ν is 1420.4058 MHz, which corresponds to a wavelength of 21 cm. 65
- 66. Next, we consider the dipole-dipole term in our last expression for HHF. Also, we have I•J=½(F2 −I2 −J2) and 〈F,mF|I•J|F,mF〉=½[F(F+1)−I(I+1)− j( j+1]. Again, only the diagonal matrix elements are non-zero. Therefore the energies are given by: lll )½)(1( 11 33 o 3 3 ++ = na Z r For hydrogen, we use from the Table for 〈rk〉: )]1()1()1([ )½)(1(33 o 3 +−+−+ ++ = IIjjFF jjna Z E B l hγµ 2017 MRT 66
- 67. For the state 22P1/2, we have Z=1, j=1/2, I=½, n=2 and l=1, which gives: 2017 MRT which is smaller, by a factor of 24, than the splitting due to the Fermi (1901-1954) contact interaction (i.e., the term geµBgNµN way above.) ( ) ( ) −=∆ = − = = 3 o 3 o 3 o 99 2 0 6 1 1 18 1 a E F a F a E B B B h h h γµ γµ γµ Similarly, for the state 22P3/2, we have Z=1, j=3/2, I=½, n=2 and l=1, which gives: ( ) ( ) =∆ = − = = 3 o 3 o 3 o 945 4 1 18 1 2 30 1 a E F a F a E B B B h h h γµ γµ γµ 67
- 68. The quadrupole moment of a nucleus is a measure of the departure of the mean distribution of nuclear charge from spherical symmetry. It is positive for a distribution which is prolate ellipsoid (e.g., like a football), negative for an oblate (e.g., like a door knob), and zero for a spherically symmetric distribution. Some nuclei that possess quadrupole moments are 2H (+), 14N (+), 17O (−), and 35Cl (−). Referring to a coordinate system (see Figure) whose origin is located within the nucleus, the electrostatic interaction, W, between a single electron (with charge e) and a nucleus containing Z protons is: ∑= = Z e W 1p pe 2 rr −−−− Coordinate system for the equation W=Σp (e2/|re – rp|) . ∑ ∑ ∞ = −= + > < + = 0 1eepp * pe ),(),( 12 1 π4 1 l l l l l ll l ll lm mm r r YY ϕθϕθ rr −−−− Another variation of this equation is obtained by writing: 2017 MRT From a relation we obtained earlier – that is 1/|r1 −−−− r2|= ΣlΣml [4π/(2l +1)](r< l/r> l+1)Yl ml*(θ1,ϕ1)Yl ml (θ2,ϕ2) – we get: in which re is the position vector of the electron, rp is the position vector of the p-th proton, and the sum is taken over all Z protons. ∑−= =•=• l l ll llll l ll m mm YY ),(),(),(),( eepp * ee )( pp )()( e )( p ϕθϕθϕθϕθ YYYY ∑ ∞ = + > < • + = 0 1 )( p )( e pe 12 1 π4 1 l l l ll l r r YY rr −−−− Substitution in 1/|r1 −−−− r2|above yields: z y x rp re |re − rp | e Z eO 68
- 69. For an electron that does not penetrate the nucleus, re >rp, so that W above becomes: 2017 MRT such that: ∑∑ ∑= ∞ = −= ++ −= Z m mm r r YYeW 1p 0 1 e p eepp *2 ),(),( 12 1 π4 l l l l l ll l ll l ϕθϕθ When l=0, W becomes: ( )0 e 2 )0()0( Coulomb =−=•= l r eZ W UQ It is possible to express W more compactly by writing: ),(),(),(),()1(),(),( ee )( pp )( eeppeepp * ϕθϕθϕθϕθϕθϕθ ll l l ll l l ll l lll l ll YY •=−= ∑∑ −= − −= m mmm m mm YYYY 1 e ee )()( 1p ppp )()( 1 ),( 12 π4 ),( 12 π4 + = + −≡ + ≡ ∑ l lllll ll r ere Z ϕθϕθ YUYQ and We then have: )3()2()2()1()1()0()0( 0 )()( OW +•+•+•=•= ∑ ∞ = UQUQUQUQ l ll which is the ordinary Coulomb interaction with Z being the sum over the protons.* * For a nucleus of finite size, there is a correction term to be added to W (i.e., (2π/3)Ze2|ψ100 |2 〈R2〉 in which 〈R2 〉 is the mean square charge radius of the nucleus and e2|ψ 100 |2 is the electronic charge density at the nucleus.) 69
- 70. The term with l=1 in W=ΣlQ(l)•U(l) vanishes (i.e., Q(1)•U(1) =0) because it corresponds to the interaction between a nuclear electric dipole moment and the electric field established by the electrons. 2017 MRT We will now suppose that the electronic state is characterized by angular momentum quantum numbers j,mj; the nuclear state by I,mI; and when the two angular momenta are coupled, the quantum numbers are I, j, F,mF. We shall compute the interaction energy associated with HQ in the latter representation. The pertinent expression is: which are the matrix elements of the scalar product T(k)•U(k) in the coupled representation. In the present context, this expression becomes: The next term, with l=2, is the electric quadrupole interaction: )2()2( Q UQ •=W 2 )( 21 )( 1 12 21 21 )()( 21 12 )1(,;,,;, jjjj kjj jjj mjjjmjjj kk mmjj jjj j kk j jj ′′ ′′ −=′′′′• ′′ ′++ UTUT δδ jjII Ij FjI mFjImFjI FF mmFF IjF FF ′ −= ′′• ′′ ++ )2()2( )2()2( 2 )1( ,;,,;, 1 UQ UQ δδ where the quantities {: : :} are called 6j-symbols and they are usually found in spec- ialized tables(Refs) and 〈I||Q(2)||I〉〈 j||U(2) || j〉 are called the reduced matrix elements. 70
- 71. In such tables we find (e.g., with s=a+b+c and X=a(a+1)−b(b+1)−c(c+1)): 2017 MRT where X=I(I+1)+ j( j+1)−F(F+1). 2/1 )]32)(22)(12(2)12)(32)(22)(12(2)12[( )]1()1(4)1(3[2 )1( 2 +++−+++− ++−+ −= cccccbbbbb ccbbXX bc cba s Themainproblemthen is to evaluatethe reduced matrix elements 〈I||Q(2)||I 〉〈 j ||U(2) || j〉. These 6j-symbols are invariant under: 1) an interchange of columns and; 2) an interchange of any two numbers in the bottom row with the corresponding two numbers in the top row. )32)(22)(12(2)12)(32)(22)(12(2)12( )]1()1(4)1(3[2 )1( 2 +++−+++− ++−− −= ++ jjjjjIIIII jjIIXX Ij FjI jIF So, by just interchanging the first and third columns, we find ours: 71
- 72. First consider 〈I ||Q(2)||I 〉. This quantity can be related to the nuclear quadrupolar moment Q which is defined by: 2017 MRT Since: )2( 1p 2 ppp 0 2 p 2 p 2 p 2 ),( 5 π4 2)3( O Z Q e rYrz ==− ∑∑ = ϕθ or: ImIrzImIQ II =−== ∑ ,)3(, p 2 p 2 p ImIQImIQe IOI === ,, 2 1 )2( It is now possible to invoke the Wigner-Eckart theorem: II mm II ImIQImI II mI IOI I )2()2( 0 2 )1(,, Q − −=== − and, on setting mI =I and evaluating the 3j-symbols, we get: IIIIIIIImIQImI IOI )2()2( )32)(1)(12)(12(,, Q+++−=== Qe II IIII II )12( )32)(32)(1)(12( 2 1)2( − ++++ =Q where the second equality comes from(i.e.,forl=2)Q(2)=eΣp√(4π/2⋅2+1)Yp (2)rp 2,we have: 72
- 73. The computation of 〈 j ||U(2) || j 〉 proceeds in analogous fashion. This time we define a quantity eQ/2 as: 2017 MRT where, from (i.e., for l=2) U(2) =−e√(4π/2⋅2+1)Ye (2) /re 2+1, we get: − −=−= 5 e 2 e 2 e 3 e ee 0 2 )2( 3 2 11 ),( 5 π4 r rz e r YeUO ϕθ We note that ∂2(−e/r)/∂z2 =−e(3z2 −r2)/r5 is the zz (diadic) component of the electric field gradient tensor produced by an electron at a point whose coordinated with respect to the electron are [x,y,z]. Since the origin of the coordinate system (see previous Figure) has been positioned at the nucleus 2UO (2) in the equation above is the zz component of the electric field gradient tensor at the nucleus produced by an electron at re, or UO (2) = ½(∂2V/∂z2)O =½Vzz where V is the potential due to the electron and the second derivative is evaluated at the origin O (i.e.,at nucleus).We then have eq=〈 j,mj =j|Vzz | j,mj =j〉=〈Vzz〉 which is the average (or expectation value) of Vzz taken over the electronic state | j, j〉. jmjUjmjQe jOj === ,, 2 1 )2( qe jj jjjj jj )12( )32)(32)(1)(12( 2 1)2( − ++++ =U Again, the use of the Wigner-Eckart theorem leads to the result: 73
- 74. On substituting the key results into 〈I, j;F,mF |Q(2) ••••U(2) |I, j;F,mF 〉 one obtains: 2017 MRT in which e2qQ is known as the quadrupole coupling constant and X is the same quantity we identified earlier (i.e., X=I(I+1)+ j( j+1)−F(F+1)). The quadrupole coupling constant may be positive or negative. This is the electric quadrupole interaction Hamiltonian. ++−− −− =′′• )1()1()1( 4 3 )12()12(2 ,;,,;, 2 )2()2( jjIIXX jjII qQe mFjImFjI FF UQ )1()1()1( 4 3 ,;,)( 2 3 )(3,;, 222 ++−−=′′ −•+• jjIIXXmFjImFjI FF JIJIJI which, when compared to our previous result for 〈I, j;F,mF |Q(2) ••••U(2)|I, j;F,mF 〉 gives: −•+• −− =•= 222 2 )2()2( Q )( 2 3 )(3 )12()12(2 JIJIJIUQ jjII qQe H The above equation can be cast into another form.From F=I++++J and −2I•J=I2 +J2 −F2, as well as 〈I, j;F,mF |−2I••••U|I, j;F,mF 〉=I(I+1)+ j( j+1)−F(F+1)=X, we get: 74
- 75. Now (without proof – c.f. M. Weissbluth) assuming axial symmetry, Vxx =Vyy, we have: 2017 MRT where Vzz =eq. For this case there are only diagonal elements with twofold degenerates in mI, that is, states with ±mI have the same energy. )]1(3[ )12(4 ,, )]3([ )12(4 2 2 QQ 22 Q +− − == − − = IIm II qQe mIHmIE IIV II eQ H III zzz i. When I=1, the energies are: ( ) ( ) =− ±=+ =−=+− − = 0 2 1 1 4 1 )23( 4 1 )]11(13[ )11.2(1.4 2 2 222 2 Q I I II mqQe mqQe mqQem qQe E I = 1 mI EQ (1/4)e2qQ±1 0 −(1/2)e2qQ and the Figure below shows the quadrupole splitting (when I=1 and e2 qQ>0). As has already been noted, the quadrupole interaction vanishes when I=0 or ½. 75
- 76. ii. For I=3/2, the energies are: 2017 MRT ( ) ( ) ±=− ±=+ = −=+− − = 2/1 4 1 2/3 4 1 4 15 3 12 1 )]12/3(2/33[ )12/3.2(2/3.4 2 2 222 2 Q I I II mqQe mqQe mqQem qQe E and the Figure below shows the quadrupole splitting (when I=3/2 and e2 qQ>0). and the Figure below shows the quadrupole splitting (when I=2 and e2 qQ<0). I = 3/2 mI EQ (1/4)e2qQ±3/2 −(1/4)e2qQ±1/2 I = 2 mI EQ −(1/8)e2qQ±1 −(1/4)e2qQ0 (1/4)e2qQ±2 iii. Finally, for I=2, the energies are: ( ) ( ) ( ) =− ±=− ±=+ =−=+− − = 0 4 1 1 8 1 2 4 1 )63( 24 1 )]12(23[ )12.2(2.4 2 2 2 222 2 Q I I I II mqQe mqQe mqQe mqQem qQe E 76
- 77. And now we remind ourselves of the The Dirac Equation chapter at end of PART IV – QUANTUM FIELDS by recalling the Dirac equation with electromagnetic coupling. We obtained the equation for an electron to order v2/c2: 2017 MRT where E′=E−moc2 and e is the electronic charge. This equation, which may be regarded as the Schrödinger equation for an electron interacting with fields describable by the potentials A and ϕ, is the starting point for discussions of atomic and molecular properties. ψϕϕ ψϕ •−•−− −•+ +=+′ p AAp ××××∇∇∇∇σσσσ∇∇∇∇∇∇∇∇ ××××∇∇∇∇σσσσ 22 o 22 o 2 23 o 4 o 2 o 488 22 1 )( cm e cm e cm p cm e c e m eE hh h 77 In the case of hydrogen energy levels En j are described by the total angular momentum by the total quantum number j=l+s=1/2,3/2,…(N.B.,l=0,1,2,…ands=½) with: with the principle quantum number n = n′+j +½ =1,2,… (n′=0,1,2,…). The electromag- netic fine structure constant is α =e2/4πhc≅1/137 and F=I++++J (i.e.,F=I++++ L++++S) which represents the vector coupling of nuclear spin I and total angular momentumJ=L++++S. ... )½)(1( )1()1()1( 4 3 ½ 11 1 2 1 33 o 2 2 2 e 22 e + ++ +−+−+ + − + +−= l h jjna IIjjFF njnn cmcmE Bjn γµ α α
- 78. This is the energy associated with the scalar potential energy, ϕ (105 cm−1). And the significance of the various terms and their energies, is indicated to within an order of magnitude (N.B., the ‘cm−1’ scale is given by the wave number 1/λ ≅ 8000 cm−1): This contains the kinetic energy (i.e., p2 /2me) and interaction term (i.e., (e/2mec)(p • A+ A • p) + e2A2/2mec2) with a field represented by a potential vector A (105 cm−1). The interaction terms are responsible or contribute to numerous physical processes among which are absorption, emission and scattering of electromagnetic waves, diamagnetism, and the Zeeman effect. 2017 MRT ϕe The spin-orbit interaction (10-103 cm−1). More precisely, itis (eh/8mec2){σσσσ •[p −−−− (e/c)A]×××× E −−−− σσσσ • E ×××× [p −−−− (e/c)A]} and it arises from the fact that the motion of the magnetic moment gives rise to an electric moment for the particle which then interacts with the electric field. This term appears in the expression of the relativistic energy: and is therefore a relativistic correction to the kinetic energy (i.e., p2/2me) (0.1 cm−1). The interaction of the spin magnetic moment (i.e., µS = 2⋅e/2me⋅h/2) with a magnetic field B= ∇∇∇∇ ×××× A (1 cm−1). Thus, it is the magnetic moment of one Bohr magneton, eh/2mec (i.e., µB = 9.2741×10−24 A⋅m2 or J/T) with the magnetic field. 2 e2 1 + Ap c e m A××××∇∇∇∇σσσσ • cm e e2 h 23 e 4 8 cm p L+−+≅+ 23 ee 2 2 e 2222 e 82 )( cm p m p cmcpcm 4 ϕ∇∇∇∇∇∇∇∇ •− 22 e 2 8 cm eh This term produces an energy shift in s-states and is known as the Darwin (1887-1962) term (< 0.1 cm−1). It is thus a correction to the direct point charge interaction due to the fact that in the representation (Foldy-Wouthuysen), the particle is not concentrated at a point but is spread out over a volume with radius whose magnitude is roughly that of a Compton wavelength, h/mec. p××××∇∇∇∇σσσσ ϕ•− 22 e4 cm eh ϕe c e m − + 2 e2 1 Ap As a combination, this term represents the interaction of a point charge with the electromagnetic field. 78
- 79. In the previous chapters we solved the Schrödinger equation exactly for a simple Coulomb potential energy function as it applies to the hydrogenic atom. In general, for any arbitrary potential, it is not possible to solve the Schrödinger equation exactly and we wish to take up in this last chapter approximation methods of particular utility for problems in which the potential energy function does not differ much (i.e., is only slightly perturbed) from that in a simple, previously solved problem. This is called perturbation theory and our goal in this chapter is to apply it to molecular structures. 2017 MRT Multi-Electron Atoms and Molecules 79 There are many cases that can be handled by perturbation theory; here we will be particularly interested in establishing procedures whereby various problems in atomic spectroscopy can be handled. For example, these would include: 1. The spin-orbit interaction which results from the interaction of the intrinsic magnetic moment of the electron about the nucleus; 2. The superposition of an externally applied magnetic field on the usual Coulomb interaction between an electron and the positively charged nucleus; 3. The problem of emission or absorption of electromagnetic radiation by atomic electrons; and 4. The electrons in a Helium atom whose wave functions would exactly correspond to the wave function of the electron in a Helium ion but for the slight disturbance or perturbation caused by the added repulsion of the two electrons; The first two are discussed in the text whereas the third deals with interactions and is considered in the Appendix. Onto the fourth one then! But first, let us refresh the postulates of quantum mechanics so as to use them in perturbation theory.
- 80. In developing the perturbation theory we will have occasion to express various wave functions in the following manner: 2017 MRT where the Cs are constants and where the ϕ s form a complete set of orthogonal functions. The orthogonality property, it will be recalled, is: ∑= n nnC ϕψ 80 where the integration is extended over all space, and the completeness property means that any arbitrary ψ can be put into this form. Let us agree to multiply each ϕ by a suitable constant so as to make it satisfy the condition: ( )mndmn ≠=∫ if0* τϕϕ 1 2 =∫ τϕ dn which is called the normalization condition. Then, for any ψ , we may calculate the coefficients Cn as follows: Multiply the equation for ψ given above by the conjugate ϕ m * and integrate over all space: m n nmnm CdCd == ∑ ∫∫ τϕϕτψϕ ** where use was made of the orthogonality and normalization conditions. Thus, given the function ψ , we can easily calculate Cm by evaluating the integral.
- 81. Let A be any Hermitian operator, with eigenvalues an and eigenfunctions ϕn: 2017 MRT It can be shown, using the Hermitian property, that any two eigenfunctions ϕn and ϕm are orthogonal, provided the eigenvalues an and am are distinct. If, however, ϕn and ϕm are two distinct eigenfunctions belonging to a single eigenvalue (i.e., the eigenvalue is degenerate in this case), then they need not be orthogonal, but they can be made so by a procedure to be explained next. nnn aA ϕϕ = 81 Note that the expression of the solution to a given wave equation in terms of a sum of wave functions which are solutions to a different wave equation is similar, for example, to the superposition of plane waves in simple Fourier analysis where any function may be represented by a sum of sinusoidal functions having specified amplitudes. See it this way: A single complicated electromagnetic disturbance can be represented as a sum, or superposition, of many simple plane waves of differing frequency whose amplitudes are found by doing Fourier analysis. Any attempt to measure the intensity of a single wave present in the summation representing the single complicated disturbance would yield a positive result with a relative intensity identical with the square of the absolute value of the computed amplitude. Similarly we may represent a given quantum mechanical state by a superposition of states of the complete orthonormal set of solutions to a wave equation! As such, it is most convenient to choose solutions to a wave equation closely corres- ponding to the actual wave equation we wish to solve or discuss.
- 82. Eigenfunctions of the wave equation having different energy eigenvalues are necessarily orthogonal, as we will now show. Let ψi and ψj be solutions of: 2017 MRT If the integration is extended over all space, we obtain: 0)( 2 * 2 2 *2 2 *2 2 *2 2 2 2 2 2 2 * =−+ ∂ ∂ + ∂ ∂ + ∂ ∂ − ∂ ∂ + ∂ ∂ + ∂ ∂ ∫ ∫ ∫ ∫ ∫ ∫ ∞ ∞− ∞ ∞− ∞ ∞− ∞ ∞− ∞ ∞− ∞ ∞− zdydxdEE m zdydxd zyxzyx ijji i jjjiii j ψψ ψ ψψψψψψ ψ h 82 0)( 2 0)( 2 * 2 *2 2 2 =−+∇=−+∇ jjjiii VE m VE m ψψψψ hh and Multiplying the terms in the first equation by ψ j * and the terms in the second by ψi on the right, and subtracting the second equation from the first, we obtain; 0)( 2 )( * 2 *22* =−+∇−∇ ijjiijij EE m ψψψψψψ h or integrating over the particle coordinates after representing the system in Cartesian coordinates, we obtain: 0)( 2 ])([ * 2 *22* =−+∇−∇ ∫∫ τψψτψψψψ dEE m d ijjiijij h
- 83. Since: 2017 MRT because of the boundary conditions on ψ, we get as a result: 1* =∫∫∫ τψψ dij 83 0 * * * * 2 *2 2 2 * = ∂ ∂ − ∂ ∂ = ∂ ∂ − ∂ ∂ ∂ ∂ = ∂ ∂ − ∂ ∂ ∞ ∞− ∞ ∞− ∞ ∞− ∫∫ i ji ji ji ji ji j xx xd xxx xd xx ψ ψψ ψψ ψψ ψψ ψψ ψ 0)( 2 * 2 =− ∫ ∫ ∫ ∞ ∞− ∞ ∞− ∞ ∞− zdydxdEE m ijji ψψ h Therefore the normalized wave functions are orthogonal, for either i= j and: or i≠ j in which case Ei ≠ Ej and: 0* =∫∫∫ τψψ dij
- 84. In the first-order perturbation theory,we start with the famous Schrödinger equation: 2017 MRT which may contain a potential energy term V which is only slightly different from the potential energy V 0 of a problem already solved. Since we should expect the solutions of the Schrödinger equation above in a perturbation problem to differ very little from the solution found with V 0, we will use the wave function for V 0 in attempting to expand the solutions to the Schrödinger equation above in terms of known functions. For a given wave function ψi, let: 02 2 0000 2 V m HEEEVVV iiiiii +∇−=′+=′+=′+= h and,, ψψψ 84 0)( 2 2 2 =−+∇ ψψ VE m h where ψ 0 i and E0 i are the solutions (i.e., eigenfunctions) and permitted energy values (i.e., energy eigenvalues) of the unperturbed Schrödinger equation: 0)( 2 000 2 02 =−+∇ iii VE m ψψ h which may be written more compactly as: 0000 iii EH ψψ = The subscript i, distinguishing the i independent energy eigenvalues for the system, takes on different values with any change in the separate eigenvalues (e.g., n,l,ml) of the wave function.
- 85. Substituting V =V 0 +V ′, Ei =E0 i +E′i and ψi =ψ 0 i +ψ ′i into the Schrödinger equation above and grouping the result in ascending order of approximation, we have: 2017 MRT This first term is zero by comparing to the unperturbed Schrödinger equation, leaving us with only the second term for a first-order approximation: 0])(-)()[()( 000000 =′′−′+′−+′−+− iiiiiiii EVEVEHEH ψψψψ assuchtermsordersecond 85 0)()( 000 =′−′+′− iiii EVEH ψψ We now expand ψ ′i in terms of the complete orthonormal set of solutions to the unperturbed Schrödinger equation, ψ 0 j. That is, we let: ∑ ∞ = =′ 0 0 j jjii a ψψ which, with first-order approximation above, gives: 0)()( 0000 =′−′+−∑ ii j jjii EVaEH ψψ Note that from H0ψ 0 i =E0 iψ 0 i, this last equation can be rewritten: 0)()( 0000 =′−′+−∑ ii j jjiij EVaEE ψψ Multiplying this last equation by ψ 0 k * on the left and integrating over all space, we get: 0)( 0*00*00*000 =′−′+− ∫∫∑ ∫ τψψτψψτψψ dEdVdaEE ikiik j jkjiij
- 86. Since the ψ 0 i s are orthonormal, all but one term in the summation is zero, and this last equation becomes: 2017 MRT If k=i, E0 i −E0 k =0 and the shift in energy, E′i, due to the perturbation is: 00 0*0 ik ik ki EE dV a − ′ = ∫ τψψ 86 0)( 0*00*000 =′−′+− ∫∫ τψψτψψ dEdVaEE ikiikkiik iVi dVE iii ′≡ ′=′ ∫ τψψ 0*0 where 〈i |V ′|i〉 spell out the diagonal matrix elements (i.e., coefficients). If k≠i, the third term is zero and the aik are given by: aii is not given by this last result but we already know its value; aii =1 approximately since ψ i ~ψ 0 i : 11 22 =−= ∑≠ij jiii aa up to and including terms of first order in the perturbation.
- 87. Note that the first-order shift in energy from the unperturbed energy level, given by E′i = ∫ψ 0 i *V′ψ 0 i dτ, is just the perturbed potential energy averaged over the unperturbed wave functions: 2017 MRT where 〈k |V ′|i〉 spell out the off-diagonal matrix elements. We obtain thus the following expressions for the energy and wave functions of the perturbed system to first order: 0 0 00 00 k ik k ki ik iiiiii EE V VEE ψψψ ∑ ∞ ≠ = − ′ +=′+= and 87 iVkdVV ikik ′≡′=′ ∫ τψψ 0*0 Note also that the effect of the perturbing potential is to give the particle a small but finite probability of occupying states of the unperturbed system other than the single unperturbed state it would be in if no perturbing potential existed. At this point we will stop but not before mentioning that there also exists the possibility of developing a second-order perturbation theory. This is needed when the first-order terms are zero or in general if a better approximation is needed, the second-order terms must be kept. We can generalize the result further then by assuming an additional correction to the potential V ′′, so that our result may be applied also to nondegenerate cases in which a further, smaller physical effect may be estimated in addition to a larger perturbing potential. For this case we would let: iiiiiiii EEEEVVVV ψψψψ ′′+′+=′′+′+=′′+′+= 000 and,
- 88. A good first-order example is to take the total potential energy for the Helium atom as: 2017 MRT with the electronic charge e, the Helium nucleus (i.e.,Z) charge 2e (i.e., 2 protons of charge +e), r1 and r2 are the electron-nucleus separations distances for each electron, and r12 is the separation of the two electrons from each other. Note that the potential above conveniently arranges as V =V1 0 +V2 0 +V ′ where V2 0 and V2 0 are the hydrogen-like potential functions of the two electrons in the nuclear Coulomb field and their interaction energy e2/r12 is treated as a perturbation V ′. If derivatives with respect to a particular electron’s position are denoted by a subscript 1 or 2, we can write the Schrödinger equation as: 12 2 2 2 1 2 22 2 1 21 r e r e r e V +−−= = ++ electronto electron electron tonucleus electron tonucleus 88 0)( 2 0 2 0 12 2 2 2 1 =′−−−+∇+∇ iiii VVVE m ψψψ h Letting a superscript zero represent the zero-order wave function, the total wave function ψ 0 i may be written as the product ψ1 0 iψ 2 0 j of the wave functions of the two electrons taken separately, where ψ1 0 i for example is a solution of: 0)( 2 0 1 0 1 0 12 0 1 2 1 =−+∇ iii VE m ψψ h or: 0 1 0 1 0 1 0 1 iii EH ψψ = and E0 i =E1 0 i +E2 0 i. The ψ 0 i are the wave functions of prior chapters (e.g.,ψnlml (r,θ,ϕ)).
- 89. The first approximation to the energy of the Helium atom is given by substituting these wave functions into Ei =E1 0 i +E2 0 i +V′ii (e.g., E1 0 i =−(meZ2e4/2h2)(1/n1 2)): 2017 MRT with n1 and n2 are the total quantum numbers of the unperturbed hydrogen-like wave functions. For the particular case of the ground state of the Helium atom, n1 =n2 =1 and l =ml =0. Each of the one-electron wave functions is an exponential times the zero-order Laguerre polynomial (a constant), so that the normalized wave functions are: ∫+ +−= τψψψψ d r e nn eZm E nnnni 0 ),(2 0 ),(1 12 2 *0 ),(2 *0 ),(12 2 2 1 2 42 e 22112211 11 2 llll h 89 22 3 0 3 3 0 3 0 )0,1(2 0 )0,1(1 21 ee ππ ρρ ψψ −− = a Z a Z where a0 =h2/mee2, ρ1 =2Zr1/a0, and the energy E1 is given by one-half the first term in the equation for Ei above. Since in spherical coordinates, the volume element is dτ = r1 2sinθ1dr1dθ1dϕ1r2 2sinθ2dr2dθ2dϕ2 the integral in Ei above becomes: ∫ ∫ ∫ ∫ ∫ ∫ ∞ ∞ −− −=′ 0 π 0 π2 0 0 π 0 π2 0 2222 2 21111 2 1 12 22 0 2 2 1 sinsin ee )π4(2 21 ϕθρθρϕθρθρ ρ ρρ dddddd a eZ E where ρ12 =2Zr12/a0. Note that the integration over a six-dimensional space, since the overall wave function is concerned with the positions of two particles.
- 90. The integrand in the last monster integral is essentially the electrostatic interaction energy between two shells of charge density exp(−ρ1) and exp(−ρ2). To begin evaluating it let us consider just the integral over ρ1. Let us consider further the potential at a point ρ due to a shell of thickness dρ1 at ρ1; it is given by: 2017 MRT and )(eπ4 e π4 111 1 12 1 1 1 ρρρρ ρ ρ ρ ρ ρ <= − − ford d 90 )(e π4e π4 11 2 1 12 1 1 1 ρρρρ ρρ ρ ρ ρ ρ >= − − ford d )]2(e2[ π4 e π4 eπ4)( 1 2 111 11 +−=+= − ∞ − ∞ − ∫∫ ρ ρ ρρ ρ ρρρφ ρ ρ ρ ρ ρ dd The total potential at ρ due to the infinite sphere of charge density exp(−ρ1) is given by: This is the potential at a point ρ due to the entire distribution of charge density exp(−ρ1).
- 91. We can now evaluate the potential energy of interaction between the distribution of charge density exp(−ρ1) and exp(−ρ2). The potential energy per unit volume of a charge density exp(−ρ2) at a point ρ2 is given by (4πρ2)[2−(ρ2 +2)exp(−ρ2)]exp(−ρ2). Hence, the second shell of charge 4πρ2 2exp(−ρ2)dρ2 has a potential energy of: 2017 MRT 91 ])2(e2[ eπ)4( 2 2 2 2 2 2 2 2 +− − − ρ ρ ρρ ρ ρ d The total electrostatic potential of the sphere of charge density exp(−ρ2) due to the sphere with charge density exp(−ρ1) is the integral over ρ2 of this last expression: 2 0 222 2 π)4( 4 5 ])2(e2[eπ)4( 22 =+−∫ ∞ −− ρρρ ρρ d Therefore our monster integral yields a result of: 2 4 e 0 2 1 24 5 24 5 h eZm a eZ E ==′ and from Ei =−(meZ2e4/2h2)(1/n1 2 +1/n2 2)+ ∫ψ 1 0* (1,0)ψ 2 0* (1,0)(e2/r12)ψ 1 0 (1,0)ψ 2 0 (1,0)dτ above: −−= ZZ em E 4 5 2 2 2 2 4 e 1 h where mee4/2h2 is the ground state energy of the Hydrogen atom and 2Z 2 times this is the unperturbed ground state energy of the two Helium electrons.
- 92. The correction to the zero-order ground state energy of the Helium atom is, as we should suspect, large, being (5/4)(Z/2Z2)=(5/8)Z ~30% of the zero-order energy, and is subtractive since the effect of the electron-electron interaction is to detract from or put up a shield reducing the electron-nucleus interaction. The approximation is found surprisingly good, however, in view of the size: The observed ground state energy of the Helium atom is 78.6 eV, the zero-order calculated energy is 108.2 eV, and the first-order calculated energy is 74.4 eV. Thus, the zero-order calculation is found to be 38% too high while the first-order calculation is within 4.2 eV of the observed value, only 5% too low. Later, using the same wave functions we will take up a slightly different method which will reduce the error to only 2% of the observed value. 2017 MRT 92 Now we are ready to consider generalize the situation where there is more than one electron in the atomic system. To do this, we first take up the problem of a system containing two identical particles and then apply the results to the two-electron Helium atom again.
