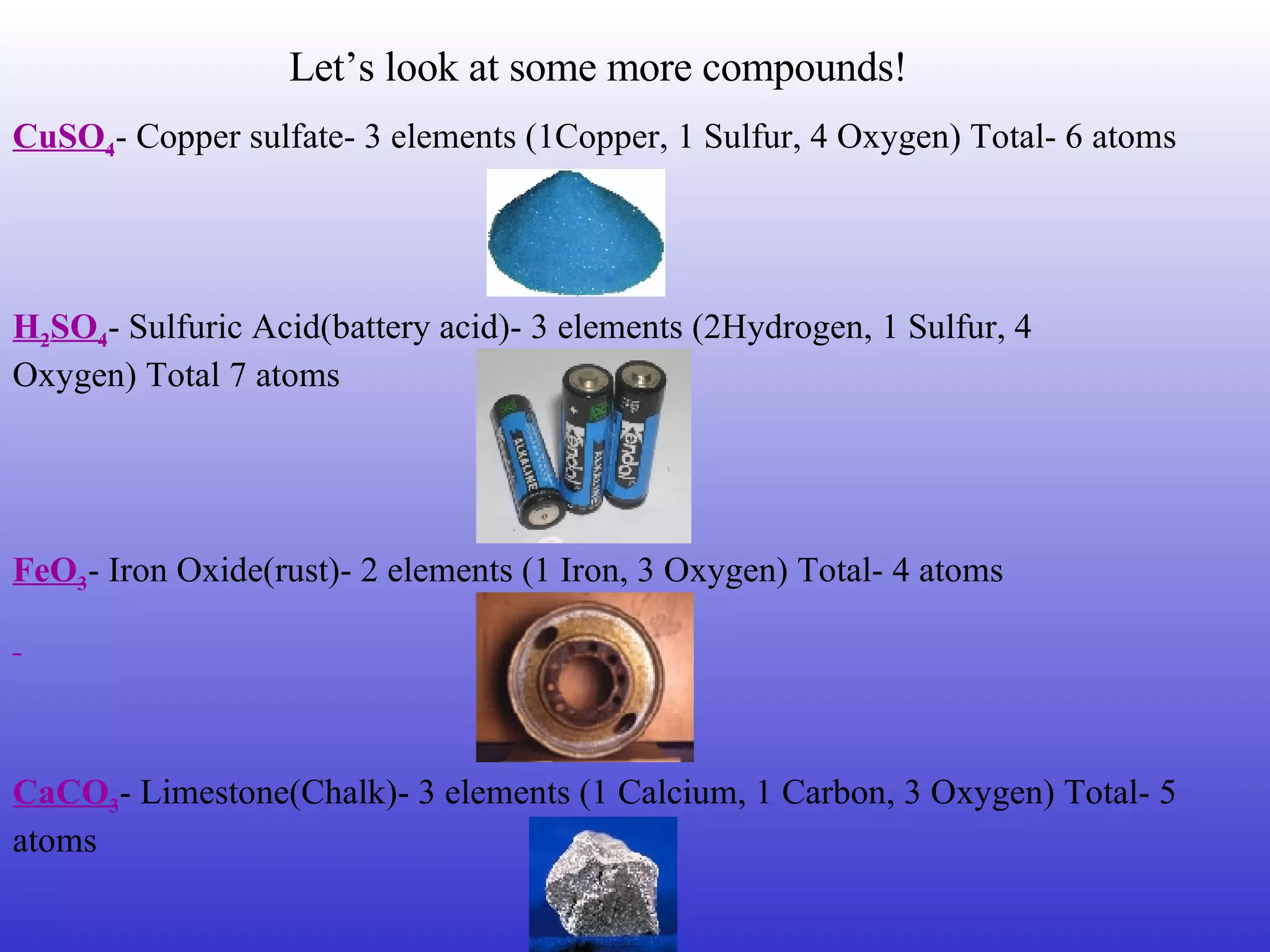
Compounds are substances composed of two or more elements that are chemically combined to form a new substance with new properties. A compound contains atoms of elements in a fixed ratio, with each particle containing the same elements in the same proportions. Mixtures are combinations of substances that are not chemically combined and can be separated using physical properties. Common compounds that make up living things include water, proteins, and DNA.