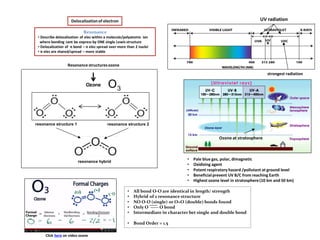
Resonance structures and ozone absorption of UV radiation
- 1. Delocalizationof electron Resonance structuresozone resonance structure 1 resonance structure 2 resonance hybrid • All bond O-O are identical in length/ strength • Hybrid of 2 resonance structure • NO O-O (single) or O=O (double) bonds found • Only O ----- O bond • Intermediate in character bet single and double bond • Bond Order = 1.5 Ozone 3O Click here on video ozone Resonance • Describe delocalization of elec within a molecule/polyatomic ion where bonding cant be express by ONE single Lewis structure • Delocalization of π bond – π elec spread over more than 2 nuclei • π elec are shared/spread – more stable • Pale blue gas, polar, dimagnetic • Oxidizing agent • Potent respiratory hazard /pollutant at ground level • Beneficialprevent UV B/C from reaching Earth • Highest ozone level in stratosphere(10 km and 50 km) Ozone at stratosphere strongest radiation UV radiation
- 2. FORMAL CHARGE (FC) Formal Charge • Tool/Model for comparing which Lewis structures is more acceptable • Treat covalent bond with equal electron distribution no electronegativity differences bet atom • Electronegative atom has negative while least electronegative atom has positive formal charge Formula formalcharge V - valence electrons of atom L – lone pair electron B – bonding electron molecule Formal charge sulfur dioxide formal charge for O V- Valence electron O = 6 L- Lone pair electron O = 4 B- Bonding electron O = 4 L + FC = 6 – (4 +2) = 0 formal charge for O V- Valence electron O = 6 L- Lone pair electron O = 6 B- Bonding electron O = 2 FC = 6 – (6+1) = -1 formal charge for O V- Valence electron O = 6 L - Lone pair electron O = 2 B - Bonding electron O = 6 FC = 6 – (2+3) = +1 All resonancestructure contributeto electronic structure. Real structure is combinationof them. Lowest FC (stable), contribute more than less stable structure. Sum of FC must be zero for neutral or equal to charge on ion.0 +1 -1 L + L + L +
- 3. Ozone Good and Bad Ozone in Strastophere • blocks UV B + C Ozone in Troposphere act as • Greenhouse gas Ozone in ground level act as • Pollutant • Photochemical Click here on ozone depletion substances ODS (phaseout) Why ozone able to absorb UV B and UV C? Breakdownof ozone – High UV radiation – Skin cancer - DNA mutation Ozone depletion UV Exposure Ozone Hole Bad SideGood Side
- 4. Ozone absorb UV radiation Ozone absorb UV B/C Ozone formation O=O Double bond O=O=O Inter mediate Bond length/pm 121 127 Bond enthalpy/kJ mol-1 498 363 Bond order 2 1.5 Dissociation by UV < 242nm < 330nm Ozone weaker bond • Absorb UV A/B (wavelength 330 nm) Bonding O2 and O3 Oxygen stronger bond • Absorb UV C (wavelength 230 nm) * Free radical -reactive species with unpair electron How ozone layer protect life on earth? Ozone Cycle O2 split by high UV to O· (radical) O· radical combine to form ozone Ozone absorb UV B/C Ozone reform again Ozone cycle Ozone created/destroyed by Chapman cycle Oxygen reform again Weaker bond in ozone broken UV O• + O2 → O3 O3 + hv → O2 + O• O3 + O• → 2O2 O2 + hv → 2O• O• + O2 → O3 O3 formation O3 destruction + + 1 2 3 4
- 5. Ozone absorb UV radiation Ozone formation O=O O=O=O Bond length/pm 121 127 Bond enthalpy/kJ mol- 1 498 363 Bond order 2 1.5 Dissociation by UV <242nm <330nm Ozone weaker bond • Absorb UV A/B (wavelength 330nm) Bonding O2 and O3 Oxygen stronger bond • Absorb UV C (wavelength 230nm) O2 split by high UV to O· (radical) O· radical combine to form ozone Ozone absorb UV B/C Ozone reform again Ozone created/destroyed by Chapman cycle Oxygen reform again Weaker bond in ozone broken UV O• + O2 → O3 O3 + hv → O2 + O• O3 + O• → 2O2 O2 + hv → 2O• O• + O2 → O3 BE O3 = 363 kJmol-1 λ need to break O3 bond Energy 1 mole, 6.02 x 1023 = 363 kJ Energy 1 photon - E = hf In nature: Ozone formation = Ozone destruction (without CFC free radical) Velocity light (c) = frequency(f) x wavelength(λ) - c = f λ • Electromagnetic waves travel at speed of light (3.00 x 108 ms-1 ) • Radiation high ↑ frequency – short ↓ wavelength • Electromagnetic radiation/photon- energy given by E = hf hc E h = plank constant = 6.626 x 10-34 Js f = frequency λ = wavelength BE O2 = 498 kJmol-1 λ need to break O3 bond Energy 1 mole, 6.02 x 1023 = 498 kJ Energy 1 photon - E = hf JE 19 23 1003.6 1002.6 363000 nm E hc 330 1003.6 1031063.6 19 834 hc E JE 19 23 1027.8 1002.6 498000 nm E hc 241 1027.8 1031063.6 19 834 hc E easier to break ! harder to break ! 1 2 3 4
- 6. Ozone absorb UV B/C CFC breaks down How CFC breaks down ? High UV B/C How CI· radical destroy ozone? ✓ Catalytic destructionof ozone Ozone Depleting Substances (ODS) How CFC destroys ozone ? CI free radicalsform CI· react O3 form CIO· radical CIO· react O· form CI· radical Net - ozone break down. Carbon Fluorine Chlorine •CI + O3 → •CIO + O2 •CIO + O• → •CI + O2 O3 + O• → 2O2 + F ׀ H – C – F ׀ CI F ׀ F – C – CI ׀ CI CI ׀ F – C – CI ׀ CI
- 7. Ozone absorb UV B/C NOx breaks down How CFC breaks down ? High UV B/C How NO· radical destroy ozone? Catalytic destructionof ozone NO· free radicals form NO· react O3 form NO2· radical NO2· react O· form NO· radical Net - ozone break down. Oxides of Nitrogen Nitrogen Dioxide (NO2) Nitrogen monoxide (nitric oxide NO) Nitrous oxide N2O Break down to form NO· free radical (unpair electron) Sources of NOx NO• + O3 → NO2• + O2 NO2• + O• → NO• + O2 O3 + O• → 2O2 Ozone DepletingSubstances(ODS)
- 8. Catalytic destruction of ozone Sourceof ODS • Halogenatedsubstance • Man-made halocarbon refrigerant,solvent, propellant • Foam-blowingagent (CFC,HCFC,freon, halon) MontrealProtocolban CFC, halon, and ODS like carbon tetrachloride and trichloroethane. CFC • Contain chlorine,fluorine atom • Extremelystablewith strong bond, long half life • Stability allow CFC to stratosphere • High UV radiation react with CFC • High UV break CFC. Free Cl radicalform – destroy O3. Trichloroflouromethane CFC-11 Dichlorodifluoromethane CFC-12 Chlorodifluoromethane HCFC-22 Why halogenated CFC used? Less harmful Very harmful Why fluorinatedis safer? CI ׀ F – C – CI ׀ CI CI ׀ F – C – CI ׀ F H ׀ F – C – CI ׀ F Bond length Bond strength C – F 138 484 C – CI 176 338 C – Br 195 276 C – I 215 238 Ozone DepletingSubstances(ODS)
- 9. Conc O3 is shown below i. O2 → 2 O∙ ii. O2 + O∙ → O3 iii. O3 → O2 + O∙ Identifywhich step is exothermic Step ii, bondforming, exothermic,energygiven off With referenceto bondingin O2 and O3, which step is most endothermic Step i and ii. Bondbreaking. Step i. O2, bondorder 2 – strong bond – more energy Step ii. O3, bondorder1.5 – weak bond– less energy Catalytic destruction of ozone Ozone form from combustion of methane. CH4 (g) + 5 O2 (g) → CO2 (g) + 2 H2O (g) + 2O2 (g) Find ∆H for rxn in kJ mol -1 = - 591 + 74 = - 517 kJ mol -1 Find ∆S, in JK-1 mol-1 Find ∆G in kJ mol-1 at 298K Deduce if rxn is spontaneous at 298K CH4(g) + 5 O2 (g) → CO2(g) + 2 H2O(g) + 2O3 (g) ∆Hf 0 - 74 0 - 393 - 241 x 2 + 142 x 2 ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) CH4(g) + 5 O2 (g) → CO2(g) + 2 H2O(g) + 2 O3 (g) S0 + 186 +205 x 5 + 213 + 188 x 2 + 237 x 2 - 74 - 591 Reactant Product 1 )tan()( 148 12111063 JKS S SSS sys sys treacproductsys + 1211 + 1063 Reactant Product syssyssys STHG 1 472 )148.0(298517 kJmolG G STHG 1 472 kJmolG CCl2F2 , cause ozone depletion.Formulate eqn for each step and explain initial step by reference to bond in CCl2F2. CCI2F2 → ∙CCIF2 + CI∙ CI∙ + O3 → CIO∙ + O2 CIO ∙ + O∙ → O2 + CI∙ CIO∙ + O3 → 2O2 + CI∙ hv
