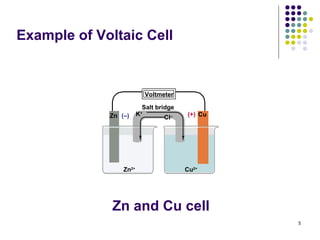
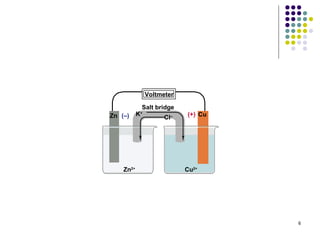
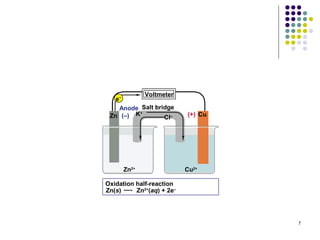
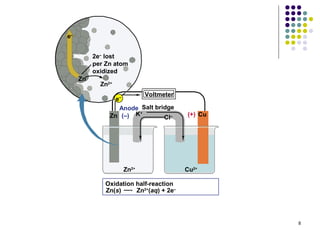
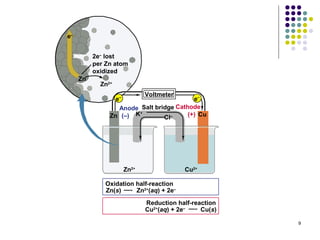
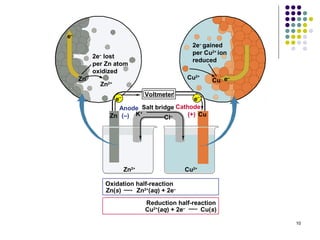
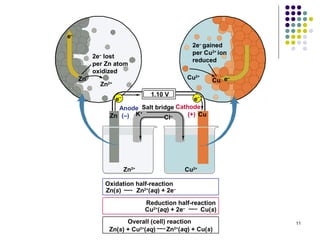
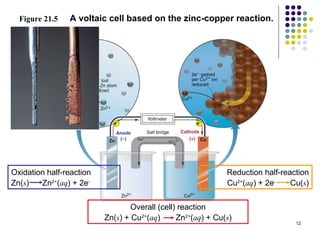
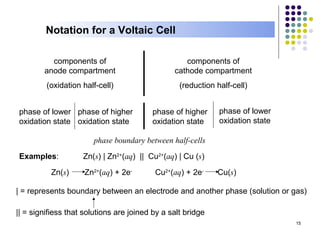
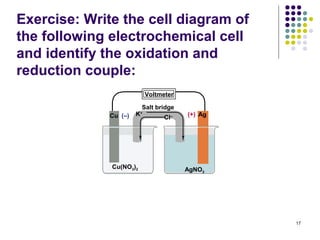
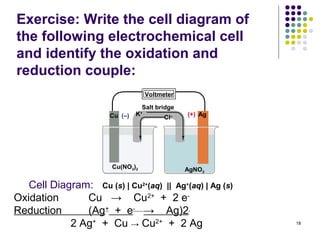
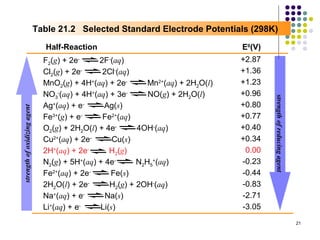
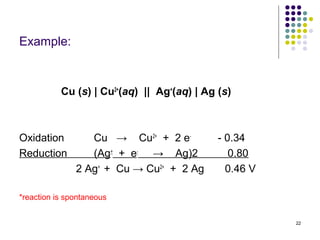
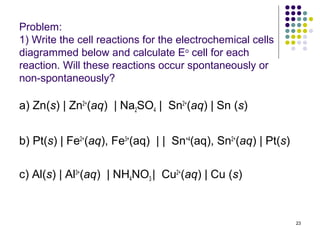
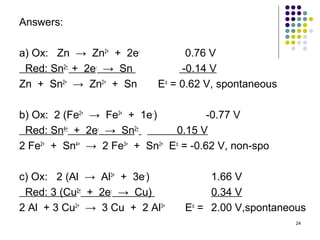
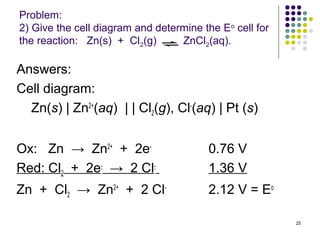
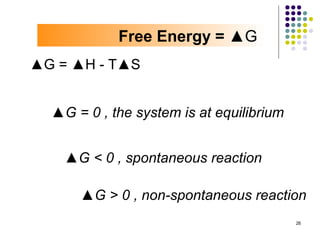
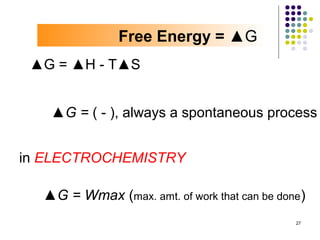
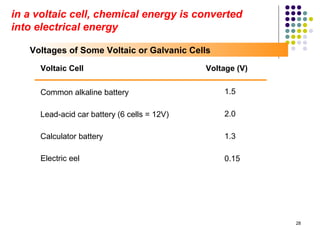
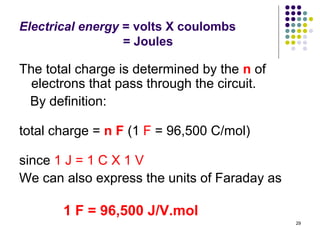
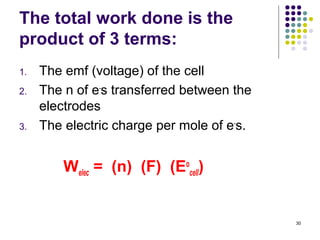
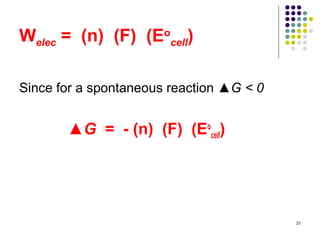
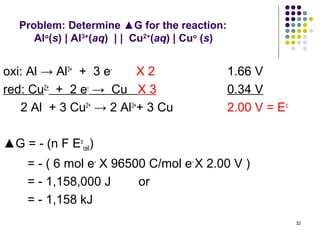
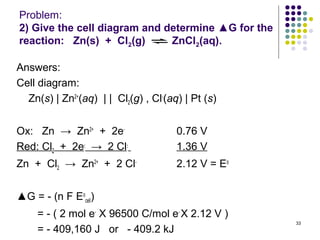
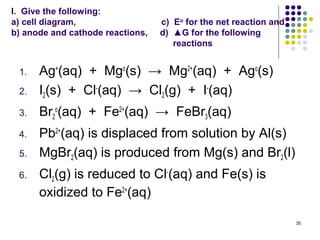
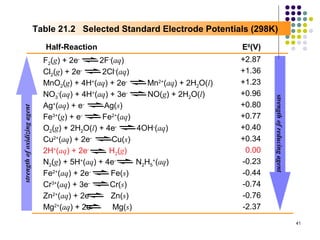
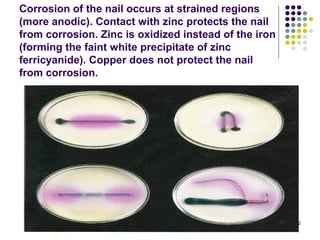
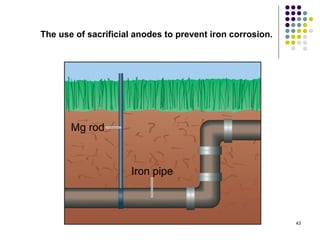
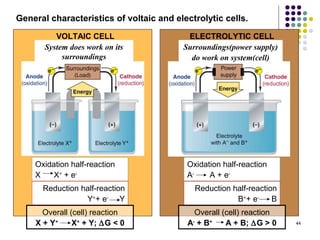
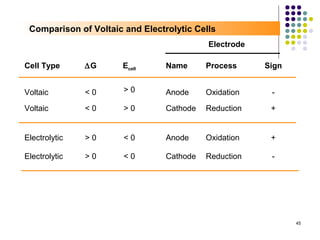
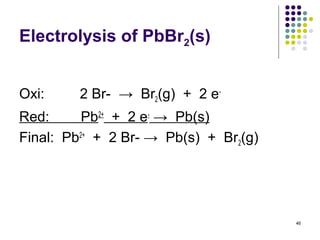
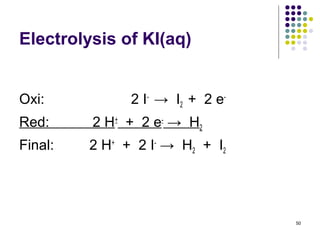
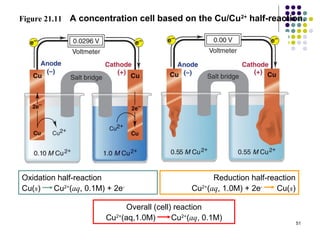
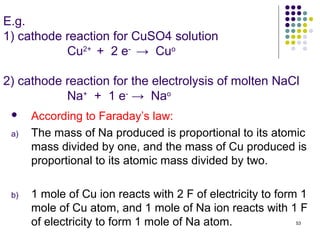
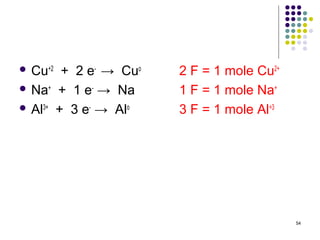
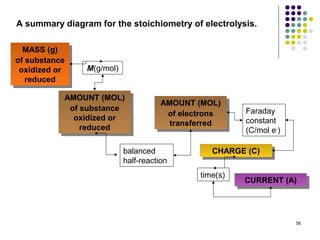
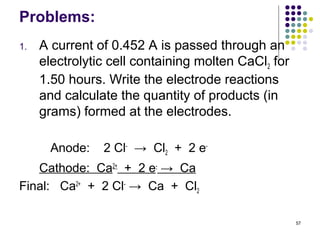
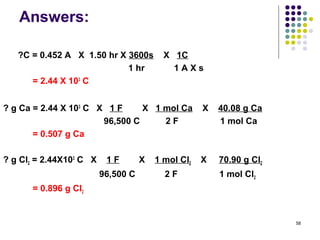
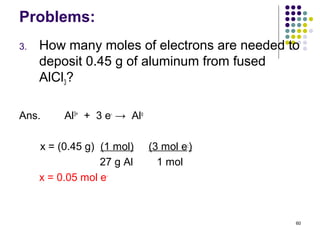
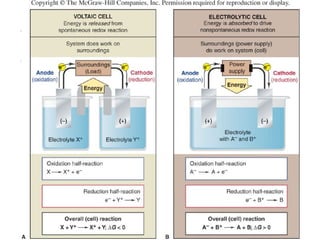
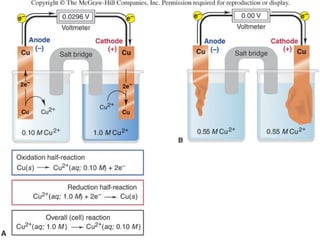
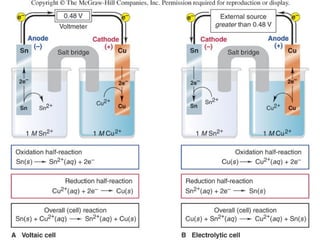
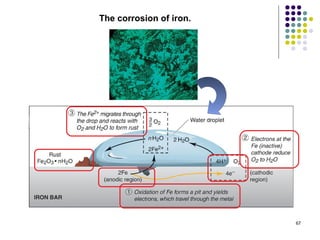
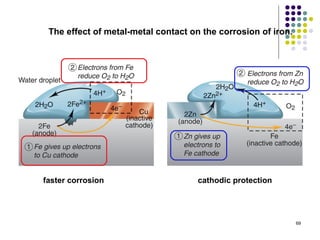
Electrochemistry deals with the interconversion of electrical and chemical energy through redox reactions. A galvanic or voltaic cell produces electricity through the spontaneous chemical change that occurs within it. A voltaic cell works because of the different abilities of materials like metals to donate or accept electrons, and the ability of electrons to flow through an external circuit. An example is a zinc-copper cell, where zinc oxidizes and copper reduces, driving electrons through the circuit.