IB Chemistry on Entropy and Laws of Thermodynamics
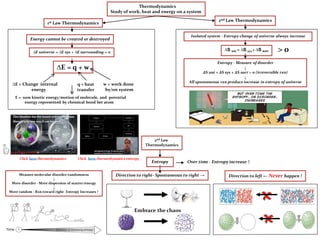
- 1. E = sum kinetic energy/motion of molecule, and potential energy represented by chemical bond bet atom ∆E = q + w ∆E = Change internal energy q = heat transfer w = work done by/on system Thermodynamics Study of work, heat and energy on a system ∆E universe = ∆E sys + ∆E surrounding = 0 1st Law Thermodynamics Entropy - Measure of disorder ↓ ∆S uni = ∆S sys + ∆S surr > 0 (irreversible rxn) ↓ All spontaneous rxn produce increase in entropy of universe 2nd Law Thermodynamics ∆S uni = ∆S sys + ∆S surr Isolated system - Entropy change of universe always increase Click here thermodynamics entropy Entropy Measure molecular disorder/randomness ↓ More disorder - More dispersion of matter/energy ↓ More random - Rxn toward right- Entropy Increases ↑ Direction to right- Spontaneous to right → 2nd Law Thermodynamics Embrace the chaos Over time - Entropy increase ↑ Direction to left ← Never happen ! Click here thermodynamics Energy cannot be created or destroyed > 0
- 2. ∆S = Entropy change Entropy Dispersal/DistributionMatter Energy Matter more disperse ↑ Entropy increases ↑ solid liquid gas spontaneous - entropy ↑ Over time - Entropy increase ↑ Phase change - sol → liq → gas ↓ Entropy increase ↑ Every energy transfer - increase entropy universe Entropy universe can only go up - never go down Entropy increase - many ways energy spread out Dispersion energy as heat - increase entropy Stoichiometry- more gas/liq in product ↓ Entropy increase ↑ T Q S Heat added ↑ Phase change Stoichiometry Embrace the chaos N2O4(g) → 2NO2(g) 1 2 2H2O(l) → 2H2 (g) + O2 (g) 1 2 3 3 More gas in product - Entropy ↑ Heat added ↑ Entropy Measure molecular disorder/randomness ↓ More disorder - More dispersion of matter/energy ↓ More randon - Rxn towards right- Entropy Increases ↑ Liq more disorder than solid Gas more disorder than liq kinetic energy distributed over wide range Q = heat transfer T = Temp/K Distribution matter in space Distribution energy bet particles Direction to left ← Never happen !Direction to right- Spontaneous to right →
- 3. Statistical Entropy Entropy Measure molecular disorder/randomness ↓ More disorder - More dispersion of matter/energy ↓ More random - Entropy Increases ↑ 1st Law Thermodynamics - Doesn't help explain direction of rxn ∆S uni> 0 (+ve) → More disorder - spontaneous ∆S uni < 0 (-ve) → More order - non spontaneous Change sol → liq → gas - Higher entropy Greater number particles in product - Higher entropy More complex molecule - More atoms bonded - Higher entropy Higher temp - Vibrate faster - More random - Higher entropy Why gas mixes and not unmix? Why heat flow from hot to cold? Entropy Notes on Entropy 1st Law Thermodynamics 2nd Law Thermodynamics Energy cannot be created or destroyed Transfer from one form to another ∆E universe = ∆E sys + ∆E surrounding = 0 Isolated system ↓ ∆S uni always increase ∆E = q + w Method to calculate entropy Number microstates Thermodynamic Entropy Heat + Temp involved Gas mixesSolution diffuse Heat flow hot →cold X X X ∆E = internal energy q = heat transfer w = work done ∆S = Entropy universe ∆S = Entropy system ∆S = Entropy surrounding ∆S uni = ∆S sys + ∆S surr Law Thermodynamics 1 2 ∆S = Entropy uni WkS ln ∆S = Entropy change k = boltzmann constant W = Microstate Click here statistical entropy Click here thermodynamics entropy Why solution diffuse and not undiffuse? Unit - J mol -1 K-1 surrsysuni SSS ∆S = Entropy sys and surr
- 4. 1st Law Thermodynamics - Doesn't help explain direction of rxn ∆S uni> 0 (+ve) → More disorder - spontaneous ∆S uni < 0 (-ve) → More order - non spontaneous Change sol → liq → gas - Higher entropy Greater number particles in product - Higher entropy More complex molecule - More atoms bonded - Higher entropy Higher temp - Vibrate faster - More random - Higher entropy Measure molecular disorder/randomness ↓ More disorder - More dispersion of matter/energy ↓ More random - Entropy Increases ↑ Isolated system ↓ ∆S uni always increase Entropy Why gas mixes and not unmix? Why heat flow from hot to cold? Notes on Entropy 1st Law Thermodynamics 2nd Law Thermodynamics Energy cannot be created or destroyed Transfer from one form to another ∆E universe = ∆E sys + ∆E surrounding = 0 ∆E = q + w Gas mixesSolution diffuse Heat flow hot →cold X X X ∆E = internal energy q = heat transfer w = work done ∆S = Entropy universe ∆S = Entropy system ∆S = Entropy surrounding ∆S uni = ∆S sys + ∆S surr Law Thermodynamics 3rd Law Thermodynamics Unit - J mol -1 K-1 Standard Molar Entropy, S0 Entropy perfectly crystal at 0K = 0 Std molar entropy, S0 ↓ S0 when substance heated from 0K to 298K Std state - 1 atm / 1M sol Temp = 298K Std Molar Entropy/S0 S0 at 298 /JK-1 mol-1 Fe (s) + 27 H2O (s) + 48 Na (s) + 52 H2O (l) + 69 CH3OH (l) + 127 H2 (g) + 130 H2O (g) + 188 CO2 (g) + 218 Solid - Order ↓ Entropy Lowest Liq - Less order ↓ Entropy Higher Gas - Disorder ↓ Entropy Highest Entropy highest Why solution diffuse and not undiffuse?
- 5. Entropy Why gas mix and not unmix?Why solution diffuse and not undiffuse? Why heat flow from hot to cold? Gas mixesSolution diffuse Heat flow hot →cold X X X Unit - J mol -1 K-1 Standard Molar Entropy, S0 Entropy perfectly crystal at 0K = 0 ↓ S0 when substance heated from 0K to 298K Std state - 1 atm / 1M sol Temp = 298K Std Molar Entropy/S0 S0 at 298 /JK-1 mol-1 Fe (s) + 27 H2O (s) + 48 Na (s) + 52 H2O (l) + 69 CH3OH (l) + 127 H2 (g) + 130 H2O (g) + 188 CO2 (g) + 218 Solid - Order ↓ Entropy Lowest Liq - Less order ↓ Entropy Higher Gas - Disorder ↓ Entropy Highest Entropy highest Entropy Standard Molar Entropy, S0 Depend on Temp increase ↑ - Entropy increase ↑ Physical/phase state Dissolving solid Molecular mass Click here thermodynamics entropy Ba(OH)2 Temp Temp/K 273 295 298 S0 for H2 + 31 + 32 + 33.2 Sol → Liq → Gas - Entropy increase ↑ State solid liquid gas S0 for H2O + 48 + 69 + 188 entropy increase ↑ entropy increase ↑ Depend on Substance NaCI NH4NO3 S0 for solid + 72 + 151 S0 for aq + 115 + 260 More motion - entropy increase ↑ Higher mass - entropy increase ↑ Substance HF HCI HBr Molar mass 20 36 81 S0 + 173 + 186 + 198 S0 = 0 at 0K All sub > 0K, have +ve S0
- 6. Entropy perfectly crystal at 0K = 0 ↓ S0 when substance heated from 0K to 298K Entropy Why gas mix and not unmix?Why solution diffuse and not undiffuse? Why heat flow from hot to cold? Gas mixesSolution diffuse Heat flow hot →cold X X X Unit - J mol -1 K-1 Standard Molar Entropy, S0 Std state - 1 atm / 1M sol Temp = 298K Std Molar Entropy/S0 S0 at 298 /JK-1 mol-1 H2O (s) + 48 Na (s) + 52 H2O (l) + 69 CH3OH (l) + 127 H2O (g) + 188 CO2 (g) + 218 Solid - Order ↓ Entropy Lowest Liq - Less order ↓ Entropy Higher Gas - Disorder ↓ Entropy Highest Entropy highest Entropy Standard Molar Entropy, S0 Depend on Temp increase ↑ - Entropy increase ↑ Physical/phase state Dissolving solid Molecular mass Temp Temp/K 273 295 298 S0 for H2 + 31 + 32 + 33.2 Sol → Liq → Gas - Entropy increase ↑ State solid liquid gas S0 for H2O + 48 + 69 + 188 entropy increase ↑ entropy increase ↑ Depend on More motion - entropy increase ↑ Click here entropy notes Click here entropy, enthalpy free energy data Click here entropy CRC data booklet Higher mass - entropy increase ↑ S0 = 0 at 0K All sub > 0K, have +ve S0 Substance NaCI NH4NO3 S0 for solid + 72 + 151 S0 for aq + 115 + 260 Substance HF HCI HBr Molar mass 20 36 81 S0 + 173 + 186 + 198
- 7. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order - Less number gas ↓ Entropy surr ↑ increase - Heat release increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Combustion at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS C3H8(g) + 5O2 (g) → 3CO2(g) + 4H2O(l) ∆H = -2220 kJ at 298K C3H8(g) + 5 O2 (g) → 3 CO2(g) + 4 H2O(l) S0 +270 +205 x 5 +213 x 3 +70 x 4 1295 919 Reactant Product 1 7450 298 )2220000( JKS S T H S surr surr surr 1 )tan()( 376 1295919 JKS S SSS sys sys treacproductsys 1 70747450376 JKS SSS uni surrsysuni ∆H = -2220 kJ = -2220000J surrsysuni SSS S /JK-1 Assume Q = H at constant pressure +ve -ve spontaneous ∆Ssys = - 376 ∆Ssurr = +7450 =+ ∆Suni = + 7074 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is Combustion at 298K spontaneous?
- 8. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order - Less number gas ↓ Entropy surr ↑ increase - Heat released increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Combustion at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS CH4(g) + 2O2 (g) → CO2(g) + 2H2O(g) ∆H = - 890 kJ at 298K CH4(g) + 2 O2 (g) → CO2(g) + 2 H2O(g) S0 + 186 +205 x 2 +213 + 188 x 2 + 596 + 589 Reactant Product 1 2986 298 )890000( JKS S T H S surr surr surr 1 )tan()( 7 596589 JKS S SSS sys sys treacproductsys 1 297929867 JKS SSS uni surrsysuni ∆H = - 890 kJ = - 890 000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = - 7 ∆Ssurr = + 2986 =+ ∆Suni = + 2979 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Assume Q = H at constant pressure Is Combustion at 298K spontaneous?
- 9. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order - Liquid form ↓ Entropy surr ↑ increase - Heat released increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Condensation at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS H2O (g) → H2O(l) ∆H = - 44.1 kJ at 298K H2O (g) → H2O(l) S0 + 188 + 70 + 188 + 70 Reactant Product 1 148 298 )44100( JKS S T H S surr surr surr 1 )tan()( 118 18870 JKS S SSS sys sys treacproductsys 1 30148118 JKS SSS uni surrsysuni ∆H = -44.1 kJ = - 44 100J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = - 118 ∆Ssurr = + 148 =+ ∆Suni = + 30 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Condensation steam at 298K (25C) spontaneous? Assume Q = H at constant pressure
- 10. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↑ increase - More disorder - More gas atoms form ↓ Entropy surr ↓ decrease - Heat absorb decrease ↓ motion surr particles ↓ Heat absorb by sys from surr decrease ↓ entropy surr ↓ ∆S surr < ∆S sys (More -ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 - Atomization at 298K - Non Spontaneous surrsysuni SSS )tan()( treacprosys SSS H2(g) → 2 H(g) ∆H = + 436 kJ at 298K H2 (g) → 2 H(g) S0 + 130 + 115 x 2 + 130 + 230 Reactant Product 1 1463 298 )436000( JKS S T H S surr surr surr 1 )tan()( 100 130230 JKS S SSS sys sys treacproductsys 1 13631463100 JKS SSS uni surrsysuni ∆H = + 436 kJ = + 436 000J surrsysuni SSS S /JK-1 +ve -ve non - spontaneous ∆Ssys = +100 ∆Ssurr = - 1463 =+ ∆Suni = - 1363 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is Atomization of H2 at 298K spontaneous? Assume Q = H at constant pressure
- 11. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order - Solid form ↓ Entropy surr ↑ increase - Heat released increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S sys > ∆S surr (More -ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 - Freezing at 298K - Non Spontaneous surrsysuni SSS )tan()( treacprosys SSS H2O (l) → H2O(s) ∆H = - 6 kJ at 298K H2O (l) → H2O(s) S0 + 70 + 48 + 70 + 48 Reactant Product 1 20 298 )6000( JKS S T H S surr surr surr 1 )tan()( 22 7048 JKS S SSS sys sys treacproductsys 1 22022 JKS SSS uni surrsysuni ∆H = -6 kJ = - 6000J surrsysuni SSS S /JK-1 +ve -ve non - spontaneous ∆Ssys = - 22 ∆Ssurr = + 20 =+ ∆Suni= - 2 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is Freezing water to ice at 298K (25C) spontaneous? Assume Q = H at constant pressure
- 12. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order - Solid form ↓ Entropy surr ↑ increase - Heat released increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Freezing at 263K (-10C) - Spontaneous surrsysuni SSS )tan()( treacprosys SSS H2O (l) → H2O(s) ∆H = - 6 kJ at 263K H2O (l) → H2O(s) S0 + 70 + 48 + 70 + 48 Reactant Product 1 8.22 263 )6000( JKS S T H S surr surr surr 1 )tan()( 22 7048 JKS S SSS sys sys treacproductsys 1 8.08.2222 JKS SSS uni surrsysuni ∆H = -6 kJ = - 6000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = - 22 ∆Ssurr = + 22.8 =+ ∆Suni= + 0.8 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is Freezing water to ice at 263K (-10C) spontaneous? Assume Q = H at constant pressure
- 13. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↑ increase - More disorder - Gas form ↓ Entropy surr ↓ decrease - Heat absorb decrease ↓ motion surr particles ↓ Heat absorb by sys from surr decrease ↓ entropy surr ↓ ∆S surr < ∆S sys (More -ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 - Decomposition at 298K - Non Spontaneous surrsysuni SSS )tan()( treacprosys SSS CaCO3 (s) → CaO(s) + CO2(g) ∆H = + 178 kJ at 298K CaCO3 (s) → CaO (s) + CO2(g) S0 + 93 + 40 + 213 + 93 + 253 Reactant Product 1 597 298 )178000( JKS S T H S surr surr surr 1 )tan()( 160 93253 JKS S SSS sys sys treacproductsys 1 437597160 JKS SSS uni surrsysuni ∆H = + 178 kJ =+ 178 000J surrsysuni SSS S /JK-1 +ve -ve non - spontaneous ∆Ssys = + 160 ∆Ssurr = - 597 =+ ∆Suni= - 437 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Decomposition CaCO3 at 298K (25C) spontaneous? Assume Q = H at constant pressure
- 14. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↑ increase - More disorder - Gas form ↓ Entropy surr ↓ decrease - Heat aborb decrease ↓ motion surr particles ↓ Heat absorb by sys from surr decrease ↓ entropy surr ↓ ∆S sys > ∆S surr (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Decomposition at 1500K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS CaCO3 (s) → CaO(s) + CO2(g) ∆H = + 178 kJ at 1500K CaCO3 (s) → CaO (s) + CO2(g) S0 + 93 + 40 + 213 + 93 + 253 Reactant Product 1 118 1500 )178000( JKS S T H S surr surr surr 1 )tan()( 160 93253 JKS S SSS sys sys treacproductsys 1 42118160 JKS SSS uni surrsysuni ∆H = + 178 kJ =+ 178 000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = + 160 ∆Ssurr = - 118 =+ ∆Suni = + 42 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Decomposition CaCO3 at 1500K (1227C) spontaneous? Assume Q = H at constant pressure
- 15. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order - Less gas form ↓ Entropy surr ↑ increase - Heat release increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Oxidation at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS 2NO(g) + O2(g) → 2NO2(g) ∆H = - 114 kJ at 298K 2 NO(g) + O2 (g) → 2NO2(g) S0 + 210 x 2 + 102 + 240 x 2 + 522 + 480 Reactant Product 1 382 298 )114000( JKS S T H S surr surr surr 1 )tan()( 42 522480 JKS S SSS sys sys treacproductsys 1 33938242 JKS SSS uni surrsysuni ∆H = - 114 kJ = - 114 000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = - 42 ∆Ssurr = + 382 =+ ∆Suni = + 339 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is Oxidation of NO at 298K (25C) spontaneous? Assume Q = H at constant pressure
- 16. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order - Less gas form ↓ Entropy surr ↑ increase - Heat release increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - NH3 production at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS N2(g) + 3H2(g) → 2NH3(g) ∆H = - 92 kJ at 298K N2(g) + 3H2 (g) → 2NH3(g) S0 + 192 + 131 x 3 + 192 x 2 + 585 + 384 Reactant Product 1 308 298 )92000( JKS S T H S surr surr surr 1 )tan()( 201 585384 JKS S SSS sys sys treacproductsys 1 107308201 JKS SSS uni surrsysuni ∆H = - 92 kJ = - 92 000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = - 201 ∆Ssurr = + 308 =+ ∆Suni = + 107 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is Haber, NH3 production 298K (25C) spontaneous? Assume Q = H at constant pressure NH3
- 17. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order ↓ Entropy surr ↑ increase - Heat release increase ↑ motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - AI production at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS Fe2O3(s) + 2AI(s) → 2Fe(s) + AI2O3(s) ∆H = - 851 kJ at 298K + 143 + 105 Reactant Product 1 2855 298 )851000( JKS S T H S surr surr surr 1 )tan()( 38 143105 JKS S SSS sys sys treacproductsys 1 2817285538 JKS SSS uni surrsysuni ∆H = - 851 kJ = - 851 000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = - 38 ∆Ssurr = + 2855 =+ ∆Suni = + 2817 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is Thermite, AI production 298K (25C) spontaneous? Assume Q = H at constant pressure Fe2O3(s) + 2AI(s) → 2Fe(s) + AI2O3(s) S0 + 87 + 28 x 2 + 27 x 2 + 51
- 18. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↓ decrease - More order ↓ Entropy surr ↑ increase - Heat release increase motion surr particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr > ∆S sys (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Decomposition KCIO3 at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS 4KCIO3(s) → 3KCIO4(s) + KCI(s) ∆H = - 144 kJ at 298K + 572 + 535 Reactant Product 1 483 298 )144000( JKS S T H S surr surr surr 1 )tan()( 37 572535 JKS S SSS sys sys treacproductsys 1 44648337 JKS SSS uni surrsysuni ∆H = - 144 kJ = - 144 000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = - 37 ∆Ssurr = + 483 =+ ∆Suni = + 446 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is decomposition KCIO3 298K (25C) spontaneous? Assume Q = H at constant pressure ∆S/∆H constant over range of temp 4KCIO3(s) → 3KCIO4(s) + KCI(s) S0 + 143 x 4 + 151 x 3 + 82
- 19. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Entropy Thermodynamic Entropy Gas mixesSolution diffuse Heat flow hot →cold X X X 1 Quatitatively T H T Q Ssurr Quatitatively Entropy sys ↑ increase - More disorder ↓ Entropy surr ↑ increase - Heat release increase ↑ motion particles ↓ Heat release by sys to surr increase ↑ entropy surr ↓ ∆S surr + ∆S sys > 0 (More +ve) ↓ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 Combustion sugar at 298K - Spontaneous surrsysuni SSS )tan()( treacprosys SSS C6H12O6(s) + 6O2 (g) → 6CO2(g) + 6H2O(l) ∆H = - 2810 kJ at 298K + 821 + 1698 Reactant Product 1 9430 298 )2810000( JKS S T H S surr surr surr 1 )tan()( 877 8211698 JKS S SSS sys sys treacproductsys 1 103079430877 JKS SSS uni surrsysuni ∆H = - 2810 kJ = - 2810 000J surrsysuni SSS S /JK-1 +ve -ve spontaneous ∆Ssys = + 877 ∆Ssurr = + 9430 =+ ∆Suni = + 10307 ∆S uni > 0 (+ve) → Spontaneous ∆S uni < 0 (-ve) → Non spontaneous Is combustionsugar 298K (25C) spontaneous? Assume Q = H at constant pressure ∆S/∆H constant over range of temp C6H12O6 (s) + 6O2(g) → 6CO2(g) + 6H2O(l) S0 + 209 +102 x 6 + 213 x 6 + 70 x 6
- 20. ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 q = heat transfer Isolated system ∆S uni always increase 1st Law Thermodynamics 2nd Law Thermodynamics Energy cannot be created or destroyed Transfer from one form to another ∆E universe = ∆E sys + ∆E surrounding = 0 ∆E = q + w ∆E = internal energy w = work done ∆S = Entropy universe ∆S = Entropy system ∆S = Entropy surrounding ∆S uni = ∆S sys + ∆S surr Law Thermodynamics 3rd Law Thermodynamics Unit - J mol -1 K-1 Standard Molar Entropy, S0 Entropy perfectly crystal at 0K = 0 Std molar entropy, S0 S0 when substance heated from 0K to 298K Std state - 1 atm / 1M sol Temp = 298K spontaneous+ve -ve = S /JK-1 Exothermic - Heat released ∆Ssys = + ve ∆Ssurr = + ve ∆Suni = + ve + ∆S sys + ve , ∆S surr +ve ↓ Suni > 0 (Rxn always spontaneous) Exothermic - Heat released +ve -ve ∆Ssys = - ve + ∆Ssurr = + ve ∆Suni = + ve = spontaneous ∆S sys - ve and ∆S surr + ve ↓ Suni > 0 (Rxn spontaneous) Endothermic - Heat absorb S /JK-1 S /JK-1 ∆Ssys = + ve + ∆Ssurr = - ve = ∆Suni = + ve ∆S sys + ve and ∆S surr - ve ↓ Suni > 0 (Rxn spontaneous) spontaneous ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 C6H12O6(s) + 6O2 (g) → 6CO2(g) + 6H2O(l) ∆H = - 2810 kJ Spontaneous / non spontaneous ∆Hsys and ∆Suni 2NO(g) + O2(g) → 2NO2(g) ∆H = - 114 kJ CaCO3 (s) → CaO(s) + CO2(g) ∆H = + 178 kJ ∆H = -ve ∆H = -ve ∆H = +ve
- 21. ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni< 0 ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 q = heat transfer Isolated system ∆S uni always increase 1st Law Thermodynamics 2nd Law Thermodynamics Energy cannot be created or destroyed Transfer from one form to another ∆E universe = ∆E sys + ∆E surrounding = 0 ∆E = q + w ∆E = internal energy w = work done ∆S = Entropy universe ∆S = Entropy system ∆S = Entropy surrounding ∆S uni = ∆S sys + ∆S surr Law Thermodynamics 3rd Law Thermodynamics Unit - J mol -1 K-1 Standard Molar Entropy, S0 Entropy perfectly crystal at 0K = 0 Std molar entropy, S0 S0 when substance heated from 0K to 298K Std state - 1 atm / 1M sol Temp = 298K Non spontaneous +ve -ve = S /JK-1 Endothermic - Heat absorb ∆Ssys = + ve ∆Ssurr = - ve ∆Suni = - ve + ∆S sys + ve , ∆S surr - ve ↓ Suni < 0 (Rxn always Non spontaneous) Exothermic - Heat released +ve -ve ∆Ssys = - ve + ∆Ssurr = + ve ∆Suni = - ve = ∆S sys - ve, ∆S surr + ve ↓ Suni < 0 (Rxn Non spontaneous) Endothermic - Heat absorb S /JK-1 S /JK-1 ∆Ssys = + ve + ∆Ssurr = - ve = ∆Suni = - ve ∆S sys + ve and ∆S surr - ve ↓ Suni < 0 (Rxn Non spontaneous) Spontaneous / non spontaneous ∆Hsys and ∆Suni ∆H = + ve ∆H = + ve ∆H = - ve CaCO3 (s) → CaO(s) + CO2(g) ∆H = + 178 kJ H2O (l) → H2O(s) ∆H = - 6 kJ Non spontaneous H2(g) → 2 H(g) ∆H = + 436 kJ Non spontaneous
- 22. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Predict entropy change - quatitatively Gas mixesSolution diffuse Heat flow hot →cold X X X Reactant Product CH4(g) + 2O2 (g) → CO2(g) + 2H2O(l) CH4(g) + 2 O2 (g) → CO2(g) + 2 H2O(l) ∆Hf 0 - 74 0 - 393 - 286 x 2 S0 + 186 +205 x 2 + 213 + 70 x 2 ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react)∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) 1 )tan()( 243 596353 JKS S SSS sys sys treacproductsys 1 2990 298 )891000( JKS S T H S surr surr surr kJHsys 891)74(965 surrsysuni SSS 1 27472990243 JKS SSS uni surrsysuni Is Combustion at 298K spontaneous? Unit for ∆S - JK-1 Unit for ∆H - kJ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Combustion at 298K - Spontaneous C3H8(g) + 5O2 (g) → 3CO2(g) + 4H2O(l) C3H8(g) + 5 O2 (g) → 3 CO2(g) + 4 H2O(l) ∆Hf 0 - 104 0 - 393 x 3 - 286 x 4 S0 +270 +205 x 5 +213 x 3 + 70 x 4 Reactant Product ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) 1 )tan()( 376 1295919 JKS S SSS sys sys treacproductsys kJHsys 2219)104(2323 1 7446 298 )2219000( JKS S T H S surr surr surr surrsysuni SSS 1 70707446376 JKS SSS uni surrsysuni ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Combustion at 298K - Spontaneous 1 2
- 23. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Predict entropy change - quatitatively Gas mixesSolution diffuse Heat flow hot →cold X X X Reactant Product ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react)∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) 1 )tan()( 118 18870 JKS S SSS sys sys treacproductsys 1 148 298 )44000( JKS S T H S surr surr surr kJHsys 44)242(286 surrsysuni SSS 1 30148118 JKS SSS uni surrsysuni Is Condensation/Freezing at 298K spontaneous? ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Condensation at 298K - Spontaneous Reactant Product ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) 1 )tan()( 22 7048 JKS S SSS sys sys treacproductsys kJHsys 6)286(292 1 20 298 )6000( JKS S T H S surr surr surr surrsysuni SSS 1 22022 JKS SSS uni surrsysuni ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 -Freezing at 298K - Non Spontaneous 3 4H2O (g) → H2O(l) H2O (l) → H2O(s) H2O (g) → H2O(l) ∆Hf 0 - 242 - 286 S0 + 188 + 70 H2O (l) → H2O(s) ∆Hf 0 - 286 - 292 S0 + 70 + 48
- 24. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Predict entropy change - quatitatively Gas mixesSolution diffuse Heat flow hot →cold X X X Reactant Product ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react)∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) 1 308 298 )92000( JKS S T H S surr surr surr kJHsys 92)0(92 surrsysuni SSS 1 107308201 JKS SSS uni surrsysuni Are these rxn at 298K spontaneous? ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - NH3 production at 298K - Spontaneous Reactant Product ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) kJHsys 168)1564(1732 1 563 298 )168000( JKS S T H S surr surr surr surrsysuni SSS 1 52656337 JKS SSS uni surrsysuni ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Decomposition at 298K - Spontaneous 5 6N2(g) + 3H2(g) → 2NH3(g) N2(g) + 3H2 (g) → 2NH3(g) ∆Hf 0 0 0 - 46 x 2 S0 + 192 + 131 x 3 + 192 x 2 1 )tan()( 201 585384 JKS S SSS sys sys treacproductsys 4KCIO3(s) → 3KCIO4(s) + KCI(s) 4KCIO3(s) → 3KCIO4(s) + KCI(s) ∆Hf 0 - 391 x 4 - 432 x 3 - 436 S0 + 143 x 4 + 151 x 3 + 82 1 )tan()( 37 572535 JKS S SSS sys sys treacproductsys
- 25. 1 118 1500 )178000( JKS S T H S surr surr surr Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Predict entropy change - quatitatively Gas mixesSolution diffuse Heat flow hot →cold X X X Reactant Product ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react)∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) kJHsys 178)1206(1028 surrsysuni SSS 1 437597160 JKS SSS uni surrsysuni ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 - Decomposition at 298K - Non Spontaneous Reactant Product ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) surrsysuni SSS 1 42118160 JKS SSS uni surrsysuni ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 - Decomposition at 1500K - Spontaneous 7 8CaCO3 (s) → CaO(s) + CO2(g) CaCO3 (s) → CaO (s) + CO2(g) ∆Hf 0 - 1206 - 635 - 393 S0 + 93 + 40 + 213 1 )tan()( 160 93253 JKS S SSS sys sys treacproductsys Decomposition at 298K Decomposition at 1500K CaCO3 (s) → CaO(s) + CO2(g) CaCO3 (s) → CaO (s) + CO2(g) ∆Hf 0 - 1206 - 635 - 393 S0 + 93 + 40 + 213 1 )tan()( 160 93253 JKS S SSS sys sys treacproductsys kJHsys 178)1206(1028 Rxn Temp dependent Spontaneousat High ↑Temp Decomposition limestone CaCO3 spontaneous? 1 597 298 )178000( JKS S T H S surr surr surr
- 26. Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Predict entropy change - quatitatively Gas mixesSolution diffuse Heat flow hot →cold X X X Reactant Product ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react)∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) 1 )tan()( 22 7048 JKS S SSS sys sys treacproductsys kJHsys 6)286(292 surrsysuni SSS 1 22022 JKS SSS uni surrsysuni Is Freezing spontaneous? Unit for ∆S - JK-1 Unit for ∆H - kJ ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni < 0 - Freezing at 298K - Non Spontaneous Reactant Product ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Hsys θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) 1 )tan()( 22 7048 JKS S SSS sys sys treacproductsys kJHsys 6)286(292 1 8.22 263 )6000( JKS S T H S surr surr surr surrsysuni SSS 1 8.08.2222 JKS SSS uni surrsysuni ∆S uni = ∆S sys + ∆S surr ↓ ∆S uni > 0 -Freezing at 263K - Spontaneous 9 10H2O (l) → H2O(s) H2O (l) → H2O(s) H2O (l) → H2O(s) ∆Hf 0 - 286 - 292 S0 + 70 + 48 H2O (l) → H2O(s) ∆Hf 0 - 286 - 292 S0 + 70 + 48 Freezing at 298K (25C) Freezing at 263K (-10C) Rxn Temp dependent Spontaneousat Low ↓ temp 1 20 298 )6000( JKS S T H S surr surr surr
- 27. N2O4 (g) → 2NO2(g) Reactant Product Entropy Ice (s) Water (l) Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Method to calculate entropy Gas mixesSolution diffuse Heat flow hot →cold X X X Qualitatively Solid → Liquid NaCI(s) → Na+ (aq) + CI - (aq)N2O4 (g) → 2NO2(g) Reactant Product S θ Less More More microstates (More dispersion/random/freedom of motion) Solid → liq → gas Higher ↑ entropy Greater number particles in productMore liq/gas in product Dispersion EnergyMicrostate More dispersion of energy (Electronic, translational, rotational, vibrational, thermal) Higher entropy ∆S > 0 (+ve) - Spontaneous ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Srys θ = More - Less = +ve > 0 S θ Less More ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Ssys θ = More - Less = +ve > 0 ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Ssys θ = More - Less = +ve > 0 NaCI(s) → Na+ (aq) + CI - (aq) S θ Less More Reactant Product Qualitatively Unit - J mol -1 K-1
- 28. Reactant Product Entropy Liq N2(l) Gas N2 (g) Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Method to calculate entropy Gas mixesSolution diffuse Heat flow hot →cold X X X Qualitatively Liquid → Gas Reactant Product S θ Less More More microstates (More dispersion/random/freedom of motion) Solid → liq → gas Higher entropy Greater number particles in productMore liq/gas in product Dispersion EnergyMicrostate More dispersion of energy (Electronic, translational, rotational, vibrational, thermal) Higher entropy ∆S > 0 (+ve) - Spontaneous ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Ssys θ = More - Less = +ve > 0 S θ Less More ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Ssys θ = More - Less = +ve > 0 ∆Ssys θ = ∑Sf θ (pro) - ∑Sf θ (react) ∆Ssys θ = More - Less = +ve > 0 NH4NO3(s) → NH4 + (aq) + NO3 - (aq) S θ Less More Reactant Product Qualitatively NH4NO3 (s) → NH4 + (aq) + NO3 - (aq) Ba(OH)2 .8H2O(s) + 2NH4NO3 (s) → Ba2+ (aq) + 2NO3 - (aq)+ 2NH3 (g) + 10H2O(aq) Ba(OH)2 .8H2O(s) + 2NH4NO3 (s) → Ba2+ (aq)+ 2NO3 - (aq)+ 2NH3 (g)+10H2O(aq) Unit - J mol -1 K-1 +
- 29. Entropy decrease ↓ Entropy Why gas mixes and not unmix?Why conc solution diffuse and not undiffuse? Why heat flow from hot to cold? Predict entropy change - qualitatively Gas mixesSolution diffuse Heat flow hot →cold X X X NH4NO3 (s) → NH4 + (aq) + NO3 - (aq) C3H8(g) + 5O2 (g) → 3CO2(g) + 4H2O(g)2H2(g) + O2 (g) → 2H2O(l) 2Cu(s) + O2 (g) → 2CuO(s) Br2(l) → Br2(g) Ag+ (aq) + Br- (aq) → AgBr(s) H2(g) + CI2 (g) → 2HCI(g) Cu2+ (aq) + Zn(s) → Cu(s) + Zn2+ (aq) CaCO3 (s) → CaO(s) + CO2(g) 1 Entropy decrease ↓ Entropy decrease ↓ Entropy increase ↑ Entropy increase ↑Entropy increase ↑ Entropy increase ↑ Little change Little change 2 3 4 Reactant Product aq - more disorder solid - more order S higher ↑ S - Lower ↓ Reactant Product g - more disorder solid - more order S higher ↑ S - Lower ↓ Reactant Product Both sides equal number mol gas Reactant Product g - more disorder liq - more order S higher ↑ S - Lower ↓ Reactant Product liq- more order g - more disorder S Lower ↓ S - Higher↑ Reactant Product less g- more order more g - more disorder S Lower ↓ S - Higher↑ Reactant Product Both sides equal number mol solid Reactant Product solid- more order aq - more disorder S Lower ↓ S - Higher↑ Reactant Product solid- more order g - more disorder S Lower ↓ S - Higher↑ 5 6 7 8 9
