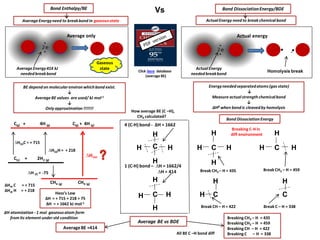
IB Chemistry on Bond Enthalpy and Bond Dissociation Energy
- 1. 4 (C-H) bond - ∆H = 1662 1 (C-H) bond – ∆H = 1662/4 ∆H = 414 ∆Hat C = + 715 ∆Hat H = + 218 H H H C(g) + 4H (g) CH4 (g) ∆Hrxn CH4 (g) ∆H(a)H = + 218 ∆H(a)C = + 715 ∆H (f) = -75 C(s) + 2H2 (g) Bond Enthalpy/BE Average Energyneed to breakbond in gaseousstate BE dependon molecularenvironwhichbond exist. ↓ Average BE values are used/ kJ mol-1 ↓ Only approximation !!!!!!! Average Energy414 kJ neededbreakbond Energyneededseparatedatoms(gas state) ↓ Measure actualstrength chemicalbond ↓ ∆Hθ when bond is cleavedby homolysis Bond DissociationEnergy/BDE Click here database (average BE) Average only ActualEnergy need to break chemical bond • .• . Homolysis break ActualEnergy neededbreakbond CH H How average BE (C –H), CH4 calculated? Actual energy Vs Hess’s Law ∆H = + 715 + 218 + 75 ∆H = + 1662 kJ mol-1 C(g) + 4H (g) H C H H H ∆H atomization - 1 mol gaseousatom form from its element under std condition Bond DissociationEnergy H C Breaking CH3 – H = 435 Breaking CH2 – H = 459 Breaking CH – H = 422 Breaking C – H = 338 H H H H HH C C C H Breaking C-H in diff environment Break CH3 – H = 435 Break CH2 – H = 459 Break CH – H = 422 Break C – H = 338 H All BE C –H bond diff Average BE vs BDE AverageBE =414 Gaseous state
- 2. ∆H(a)C = + 715 C(g) + 4CI (g) Average Energyneed to breakbond in gaseousstate 4 (C-CI) bond- ∆H = 1300 1 (C-CI) bond– ∆H = 1300/4 ∆H = 325 ∆Hat C = + 715 ∆Hat CI = + 121 CI CI CI CCI4 (g) ∆Hrxn CCI4 (g) ∆H(a)CI = + 121 ∆H (f) = -105 C(s) + 2CI2 (g) Bond Enthalpy/BE BE dependon molecularenvironwhichbond exist. ↓ Average BE values are used/ kJ mol-1 ↓ Only approximation !!!!!!! Average Energy 325 kJ neededbreakbond Energyneededseparatedatoms(gas state) ↓ Measure actualstrength chemicalbond ↓ ∆Hθ when bond is cleavedby homolysis Bond DissociationEnergy/BDE Click here database (average BE) Average only ActualEnergy need to break chemical bond • .• . Homolysis break Actual Energy neededbreakbond CCI CI How average BE (C –CI), CCI4 calculated? Actual energy Vs Hess’s Law ∆H = + 715 + 121 + 105 ∆H = + 1300 kJ mol-1 C(g) + 4CI (g) CI C CI CI CI ∆H atomization - 1 mol gaseousatom form from its element under std condition Bond Dissociation Energy CI C Breaking CCI3 – CI = 402 Breaking CCI2 – CI = 420 Breaking CCI – CI = 388 Breaking C – CI = 350 CI CI CI CI CICI C C C CI Breaking C-CI in diff environment Break CCI3 – CI = 402 Break CCI2 – CI = 420 Break CCI – CI = 388 Break C – CI = 350 CI All BE C –CI bond diff Average BE vs BDE AverageBE = 325 Gaseous state
- 3. H Bond Enthalpy/BE Average Energy414 kJ neededbreakbond Bond DissociationEnergy/BDE Click here database Average only • .• . Homolysis breakActualEnergy neededbreakbond Actual energy vs Bond DissociationEnergy H CCH3 – H = 435 CH2 – H = 459 CH – H = 422 C – H = 338 H H H H HH C C C H Breaking C-H in diff environment Break CH3 – H = 435 Break CH2– H = 459 Break CH – H = 422 Break C – H = 338 H All BE C –H bond diff Gaseous state Average BE vs BDE Average BE (C –CI), CCI4 calculated? Average BE = 414 Limitation using Average BE • Ave BE only approximation and NOT accurate • ∆Hc and ∆f more accurate ! • Ave BE used for similar bond within identicalmolecule H │ H – C – H │ H Diatomic molecule 2 atom with 1 bond/1 type bond exist ↓ No other molecule have same bond ↓ H - H, CI - CI, N = N, Br- Br, H – CI, H – Br, H – I ↓ They do not have average BE. I │ H – C – H │ H Molecules> 1 bonds,similar (C – H ) bond averageBE ( C- H ) for differentmolecule Br │ H – C – H │ H O ║ C – C – H Similar bond (C – H) in different molecule ↓ Average BE (C- H) + 414 is used + 414 + 414 + 414 + 414 Similar bond (C – CI) in different molecule ↓ Average BE (C- CI) + 325 is used H │ H – C – CI │ H I │ H – C – CI │ H Br │ H – C – CI │ I O ║ C – C – CI Molecules> 1 bonds,similar (C – CI ) bond averageBE ( C- CI ) for differentmolecule + 325 + 325 + 325 + 325
- 4. Average Energyneed to breakbond in gaseousstate Average BE ( Breaking certain bond) CH4 + CI2 → CH3CI + HCI ∆H=? C H H H H CI CI CH4 + CI2 → CH3CI + HCI ∆H=? ∆Hrxn Bond Enthalpy/BE Average Energy neededbreakbond Click here database Average only Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release ∆Hθ = ∑ Bond broken - ∑ Bond form = + 1890 – 2006 = - 116 kJ mol-1 ∆Hθ = ∑ Bond broken ∆Hθ = ∑ Bond form H │ H – C – H │ H CI - CI ∆H determinationCH4 + 2O2 → CO2 + 2H2O Average BE ( Breaking all bond) 4 (C-H) → + 414 x 4 1 (CI - CI) → + 242 Total + 1890 3 (C- H) → - 412 x 3 1 (C – CI) → - 339 1 (H - CI) → - 431 Total - 2006 Using 2 method + HCI + H │ H – C – CI │ H H │ H – C – H │ H CH3 H CI CI ∆Hθ = ∑ Bond broken 1 (C-H) → + 414 1 (CI - CI) → + 242 Total + 656 CI - CI+ ∆Hrxn ∆Hθ = ∑ Bond form 1 (C – CI) → - 339 1 (H - CI) → - 431 Total - 770 H │ H – C – CI │ H + H - CI ∆Hθ = ∑ Bond broken - ∑ Bond form = + 656 – 770 = - 114 kJ mol-1 SAME Gaseous state Same number/type bonds broken
- 5. Average Energyneed to breakbond in gaseousstate ∆Hf θ + 52 o - 85 ∆Hf θ (reactant) ∆Hf θ (product) ∆Hrxn Bond Enthalpy/BE Average EnergykJ neededbreakbond Click here database Average only Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release ∆Hθ = ∑ Bond broken - ∑ Bond form = + 2696 – 2820 = - 124 kJ mol-1 ∆Hθ = ∑ Bond broken = Energy Absorbed ∆Hθ = ∑ Bond form = Energy Released H H │ │ H – C – C – H │ │ H H H H │ │ H - C=C - H + H - H ∆H determinationC2H4 + H2 → C2H6 C C H H H H H H Average BE Std ∆Hf θ formationto find ∆H rxn 1 (C=C) → + 612 4 (C-H) → + 414 x 4 1 (H-H) → + 436 Total + 2696 1 (C-C) → - 346 6 (C-H) → - 414 x 6 Total - 2820 C2H4 + H2 C2H6 Reactants Products 2C + 3H2 ∆Hrxn θ C2H4 + H2 C2H6 Reactants Products ∆Hrxn θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) ∆Hrxn θ = - 85 - ( + 52 ) = - 137 kJ mol -1 C2H4 + H2 → C2H6 ∆H = ?C2H4 + H2 → C2H6 ∆H = ? Using 2 method Different Estimate Accurate Gaseous state
- 6. Average Energyneed to breakbond in gaseousstate ∆Hf θ + 52 o - 85 ∆Hf θ (reactant) ∆Hf θ (product) ∆Hrxn Bond Enthalpy/BE Average Energy needed break bond Click here database Average only Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release ∆Hθ = ∑ Bond broken - ∑ Bond form = + 2696 – 2820 = - 124 kJ mol-1 ∆Hθ = ∑ Bond broken ∆Hθ = ∑ Bond form H H │ │ H – C – C – H │ │ H H H H │ │ H - C=C - H + H - H ∆H determination C2H4 + H2 → C2H6 C C H H H H H H Average BE Std ∆Hf θ formation 1 (C=C) → + 612 4 (C-H) → + 414 x 4 1 (H-H) → + 436 Total + 2696 1 (C-C) → - 346 6 (C-H) → - 414 x 6 Total - 2820 C2H4 + H2 C2H6 Reactants Products 2C + 3H2 ∆Hrxn θ C2H4 + H2 C2H6 Reactants Products ∆Hrxn θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) ∆Hrxn θ = - 85 - ( + 52 ) = - 137 kJ mol -1 C2H4 + H2 → C2H6 ∆H = ?C2H4 + H2 → C2H6 ∆H = ? Using 3 method ∆Hc θ comb C2H4 + H2 → C2H6 ∆H = ? C2H4 + H2 C2H6 Reactants Products 2CO2 + 3H2O ∆Hrxn θ + 3.5O2 + 3.5O2 ∆Hc θ (product)∆Hc θ (reactant) C2H4 + H2 C2H6 ∆Hc θ -1411 -286 - 1560 ∆Hrxn θ = ∑∆Hc θ (react) - ∑∆Hc θ (pro) = ( -1411) + (-286) – (-1560) = - 137 kJ mol -1 Reactants Products Gaseous state ∆Hf θ form and ∆Hc θ comb more accurate
- 7. Average Energyneed to breakbond in gaseousstate CH4 + 2O2 CO2 + 2H2O CH4 + 2O2 → CO2 + 2H2O ∆H=? C H H H H O O O O CH4 + 2O2 → CO2 + 2H2O ∆H=? ∆Hf θ - 74 o - 393 - 286 x 2 ∆Hf θ (reactant) ∆Hf θ (product) ∆Hrxn Bond Enthalpy/BE Average Energy neededbreakbond Click here database Average only Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release ∆Hθ = ∑ Bond broken - ∑ Bond form = + 2652 – 3466 = - 814 kJ mol-1 ∆Hθ = ∑ Bond broken = Energy Absorbed ∆Hθ = ∑ Bond form = Energy Released H │ H – C – H │ H O = O O = O ∆H determinationCH4 + 2O2 → CO2 + 2H2O Average BE Std ∆Hf θ formationto find ∆H rxn 4 (C-H) → + 414 x 4 2 (O=O) → + 498 x 2 Total + 2652 2 (C=O) → - 804 x 2 4 (O-H) → - 463 x 4 Total - 3466 CH4 + 2O2 CO2 + 2H2O Reactants Products C +2O2 +2H2 ∆Hrxn θ Reactants Products ∆Hrxn θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) ∆Hrxn θ = - 965 - (- 74 ) = - 891 kJ mol -1 Using 2 method + Different Estimate Accurate H – O – H H – O – H0 = C =O + Gaseous state
- 8. Average Energyneed to breakbond in gaseousstate Reactants Products 3C2H2 → C6 H6 ∆H=? C C C C C C H H H H H H ∆Hf θ + 228 x 3 + 49 ∆Hf θ (reactant) ∆Hf θ (product) ∆Hrxn Bond Enthalpy/BE Average Energy neededbreakbond Click here database Average only Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release ∆Hθ = ∑ Bond broken - ∑ Bond form = + 5001 – 5364 = - 364 kJ mol-1 ∆Hθ = ∑ Bond broken ∆Hθ = ∑ Bond form ∆H determination3C2H2 → C6 H6 Average BE Std ∆Hf θ formationto find ∆H rxn 6 (C-H) → + 414 x 6 3 (C=C) → + 839 x 3 Total + 5001 6 (C - H) → - 414 x 6 3 (C - C) → - 346 x 3 3 (C=C) → - 614 x 3 Total - 5364 Reactants Products 6C + 3H2 ∆Hrxn θ ∆Hrxn θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) ∆Hrxn θ = + 49 - (+ 684 ) = - 635 kJ mol -1 Using 2 method Different Estimate Accurate 3 C2H2 C6H6 3C2H2 → C6 H6 ∆H=? 3C2H2 C6H6 Gaseous state
- 9. Average Energyneed to breakbond in gaseousstate 3C2H2 C6H6 Reactants Products 3C2H2 C6H6 3C2H2 → C6H6 ∆H = ? ∆Hf θ (reactant) ∆Hf θ (product) ∆Hrxn Bond Enthalpy/BE Average Energy neededbreakbond Click here database Average only Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release ∆Hθ = ∑ Bond broken ∆Hθ = ∑ Bond form Average BE Std ∆Hf θ formation Reactants Products ∆Hrxn θ 3C2H2 → C6H6 ∆H = ? Using 3 method ∆Hc θ comb Reactants Products ∆Hrxn θ + 7.5O2 + 7.5O2 ∆Hc θ (product)∆Hc θ (reactant) Reactants Products ∆H determination3C2H2 → C6 H6 3C2H2 → C6H6 ∆H = ? C C C C C C H H H H H H 6 (C-H) → + 414 x 6 3 (C=C) → + 839 x 3 Total + 5001 6 (C - H) → - 414 x 6 3 (C - C) → - 346 x 3 3 (C=C) → - 614 x 3 Total - 5364 ∆Hθ = ∑ Bond broken - ∑ Bond form = + 5001 – 5364 = - 364 kJ mol-1 3 C2H2 C6H6 6C + 3H2 ∆Hf θ + 228 x 3 + 49 ∆Hrxn θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) ∆Hrxn θ = + 49 - (+ 684 ) = - 635 kJ mol -1 3 C2H2 C6H6 6CO2 + 3H2O ∆Hc θ -1300 x 3 - 3270 ∆Hrxn θ = ∑∆Hc θ (react) - ∑∆Hc θ (pro) = 3 x (- 1300) – (-3270) = - 630 kJ mol -1 Gaseous state ∆Hf θ form and ∆Hc θ comb more accurate
- 10. Average BE 3C(g) + 6H(g) → CH2 = CH – CH3 ∆H =? C C C H H H H H H ∆Hrxn Bond Enthalpy/BE ∆Hθ = ∑ Bond broken - ∑ Bond form = 0 – 3444 = - 3444 kJ mol-1 ∆Hθ = ∑ Bond broken = Energy Absorb ∆Hθ = ∑ Bond form = Energy Released Find ∆H rxn, 3C(g) + 6H(g) → CH2 = CH – CH3 AverageBE 6 (C - H) → - 414 x 6 1 (C – C) → - 356 1 (C = C) → - 598 Total - 3444 No bond broken Find ∆H rxn, 3C(g) + 6H(g) + 2Br(g)→ CH2 = CH – CH3 3C(g) + 6H(g) + 2Br(g) → CH2 = CH – CH3 H H │ │ C = C – C - H │ │ │ H H H No bond broken ∆Hθ = ∑ Bond broken = Energy Absorb C C C H H H H H H Br Br ∆Hθ = ∑ Bond form = Energy Released ∆Hrxn Gaseous state Gaseous state 6 (C - H) → - 414 x 6 2 (C – C) → - 356 x 2 2 (C - Br) → - 284 x 2 Total - 3770 H H │ │ H - C - C – C - H │ │ │ Br Br H ∆Hθ = ∑ Bond broken - ∑ Bond form = 0 – 3770 = - 3770 kJ mol-1 Bond Enthalpy/BE Average Energyneed to breakbond in gaseousstate Average Energy needed break bond Gaseous state Click here database Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release
- 11. N2H4 + 2F2 N2 + 4HF N2H4(g) + 2F2(g) → N2(g) + 4HF (g) ∆H=? N N H H H H F F F F ∆Hf θ - 51 o 0 - 273 x 4 ∆Hf θ (reactant) ∆Hf θ (product) ∆Hrxn Bond Enthalpy/BE Average Energy needed break bond Click here database Average only Bond breaking/making involve energy Bond Breaking Heat energy absorb Bond Making Heat energy release ∆Hθ = ∑ Bond broken - ∑ Bond form = + 2031 – 3204 = - 1173 kJ mol-1 ∆Hθ = ∑ Bond broken ∆Hθ = ∑ Bond form H H │ │ N – N │ │ H H F – F F – F Average BE Std ∆Hf θ formationto find ∆H rxn 1 (N - N) → + 163 2 (F - F) → + 158 x 2 4 (N- H) → + 388 x 4 Total + 2031 1 (N N) → - 944 4 (H - F) → - 565 x 4 Total - 3204 N2H4 + 2F2 N2 + 4HF Reactants Products N2 + 2F2 + 2H2 ∆Hrxn θ Reactants Products ∆Hrxn θ = ∑∆Hf θ (pro) - ∑∆Hf θ (react) ∆Hrxn θ = - 1092 - (- 51) = - 1041 kJ mol -1 Using 2 method + N2H4(g) + 2F2(g) → N2(g) + 4HF (g) ∆H=? ∆H determinationN2H4(g) + 2F2 → N2 + 4HF ≡ N N≡ H – F H – F H – F H – F + Different Estimate Accurate Gaseous state Average Energyneed to breakbond in gaseousstate
