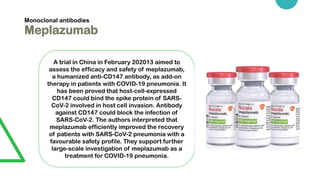
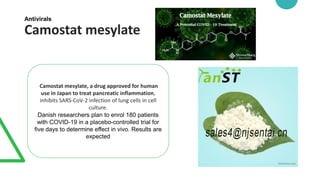
Potential medicines being studied for treating COVID-19 include monoclonal antibodies like Actemra, Kevzara, and Meplazumab which may help reduce lung inflammation. Antivirals under investigation include remdesivir, favipiravir, camostat mesylate and interferons. Other candidates include the immunosuppressants baricitinib and rintatolimod, and antibiotics like azithromycin, mefloquine hydrochloride and teicoplanin which may prevent viral entrance into cells. Povidone-iodine gargles are also being explored as a preventative measure. However, no medicine has yet been proven to prevent or cure COVID-19.