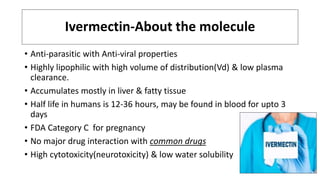
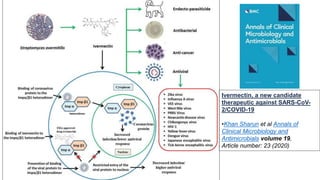
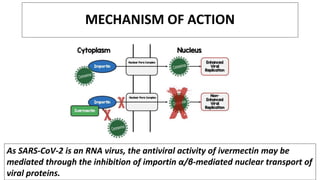
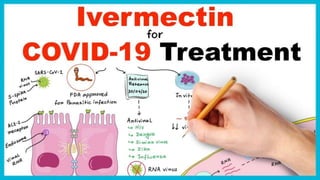
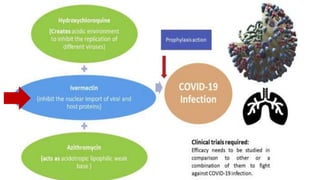
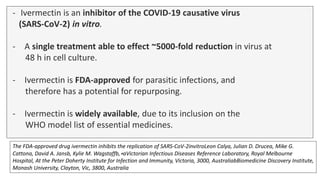
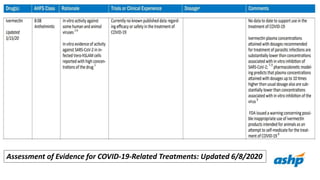
Ivermectin is an anti-parasitic drug with anti-viral properties that may help treat COVID-19. It has been shown to reduce SARS-CoV-2 virus replication in cell cultures. Ivermectin's mechanism of action involves inhibiting importin α/β-mediated nuclear transport of viral proteins. When combined with hydroxychloroquine, ivermectin and HCQ may have synergistic effects by blocking viral entry and replication. While ivermectin shows promise based on in vitro studies, its clinical efficacy for treating COVID-19 is still unpredictable as clinical trials are still needed given SARS-CoV-2 is a novel virus.