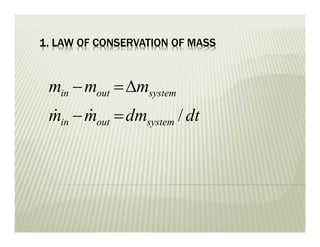
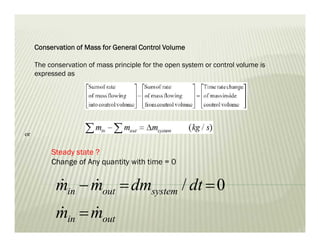
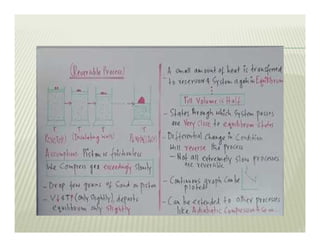
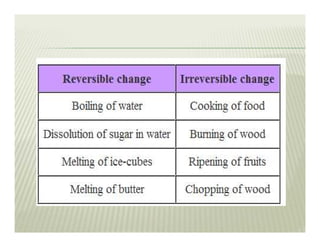
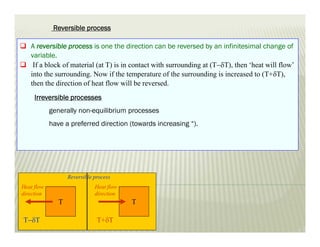
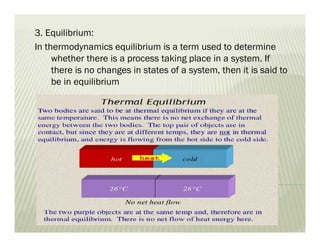
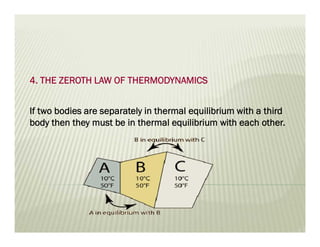
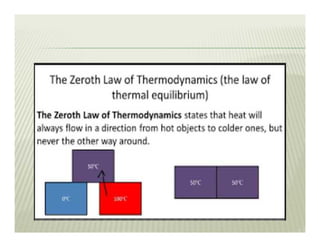
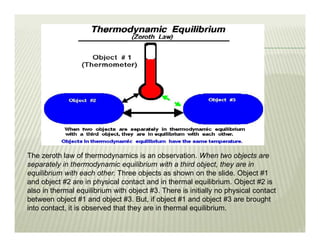
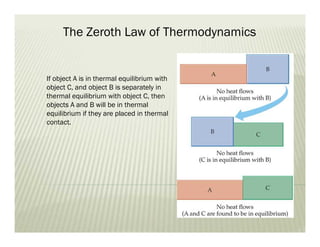
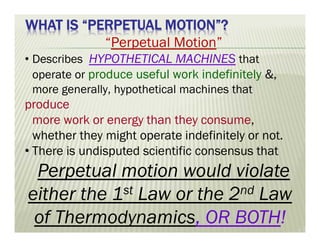
The document discusses several key concepts in thermodynamics:
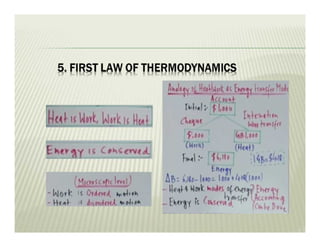
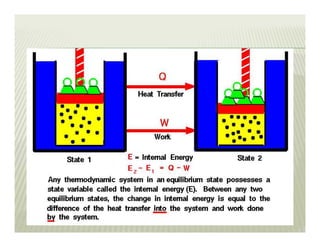
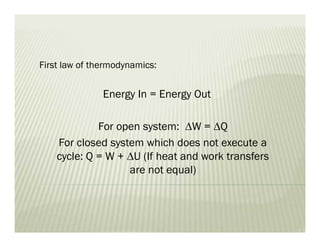
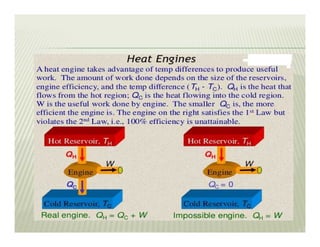
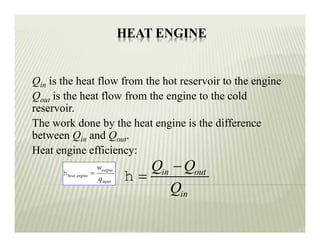
1. The first law of thermodynamics states that energy can be neither created nor destroyed, only transformed. For a cycle, the net heat transfer equals the net work transfer.
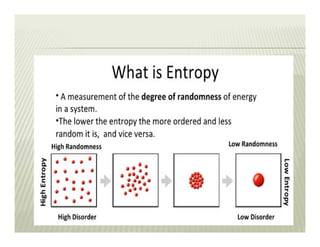
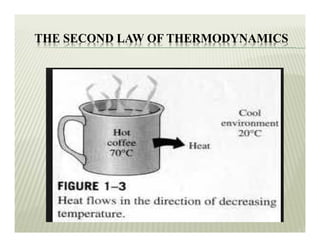
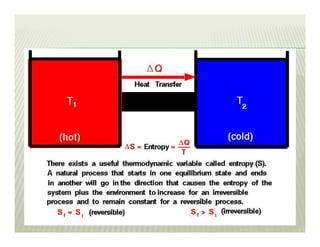
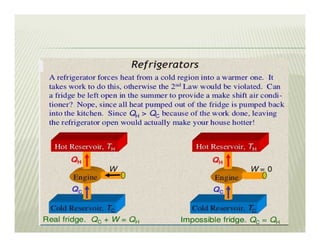
2. The second law of thermodynamics describes irreversible processes and states that it is impossible to convert all heat transfer into work. Some heat must be rejected to a cold reservoir.
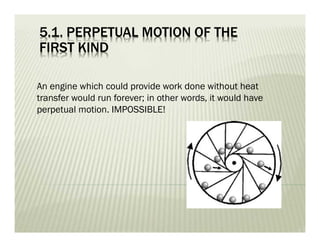
3. Perpetual motion machines that could provide work without heat transfer or produce more work than consumed would violate the first and second laws of thermodynamics.