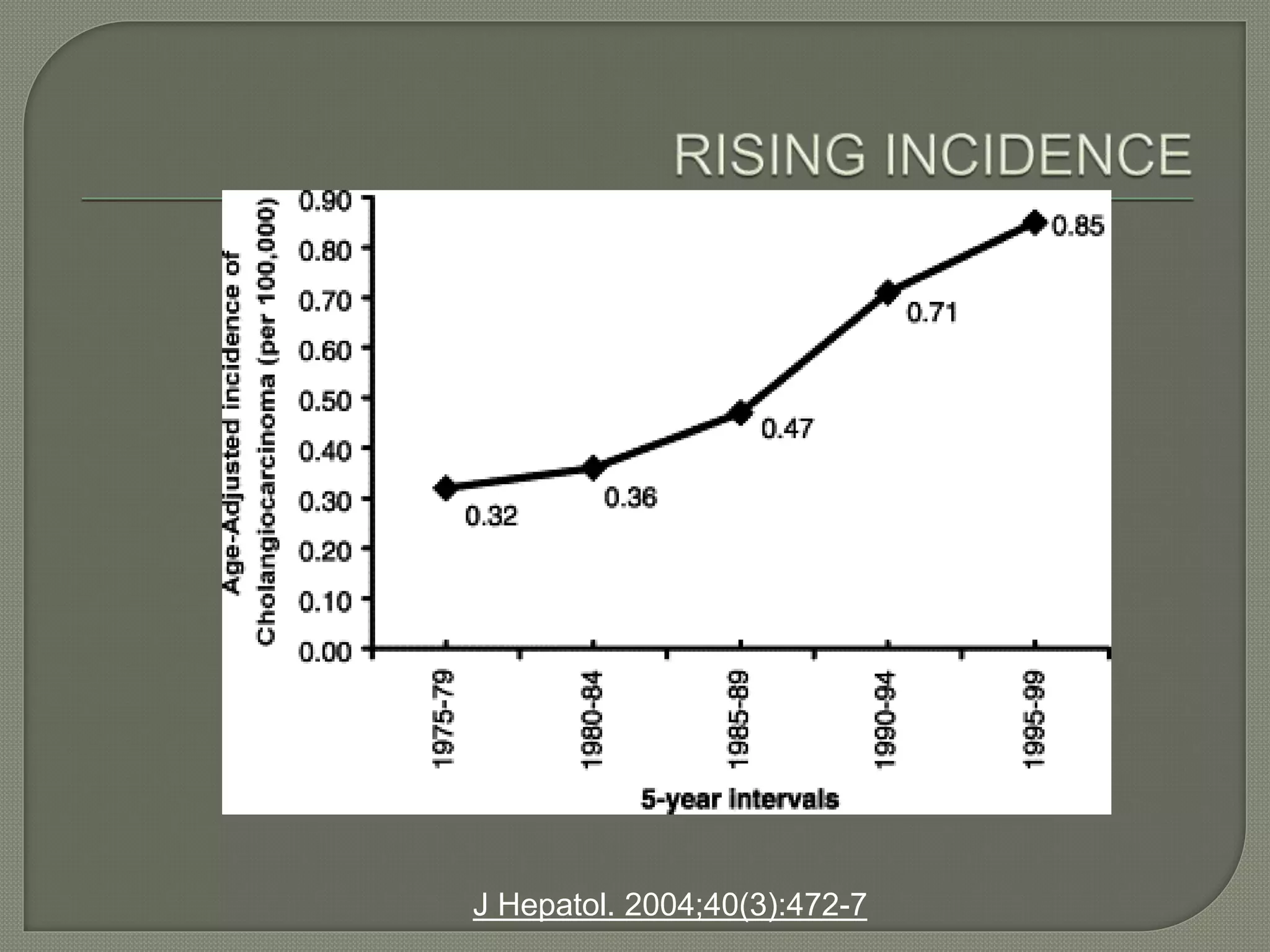
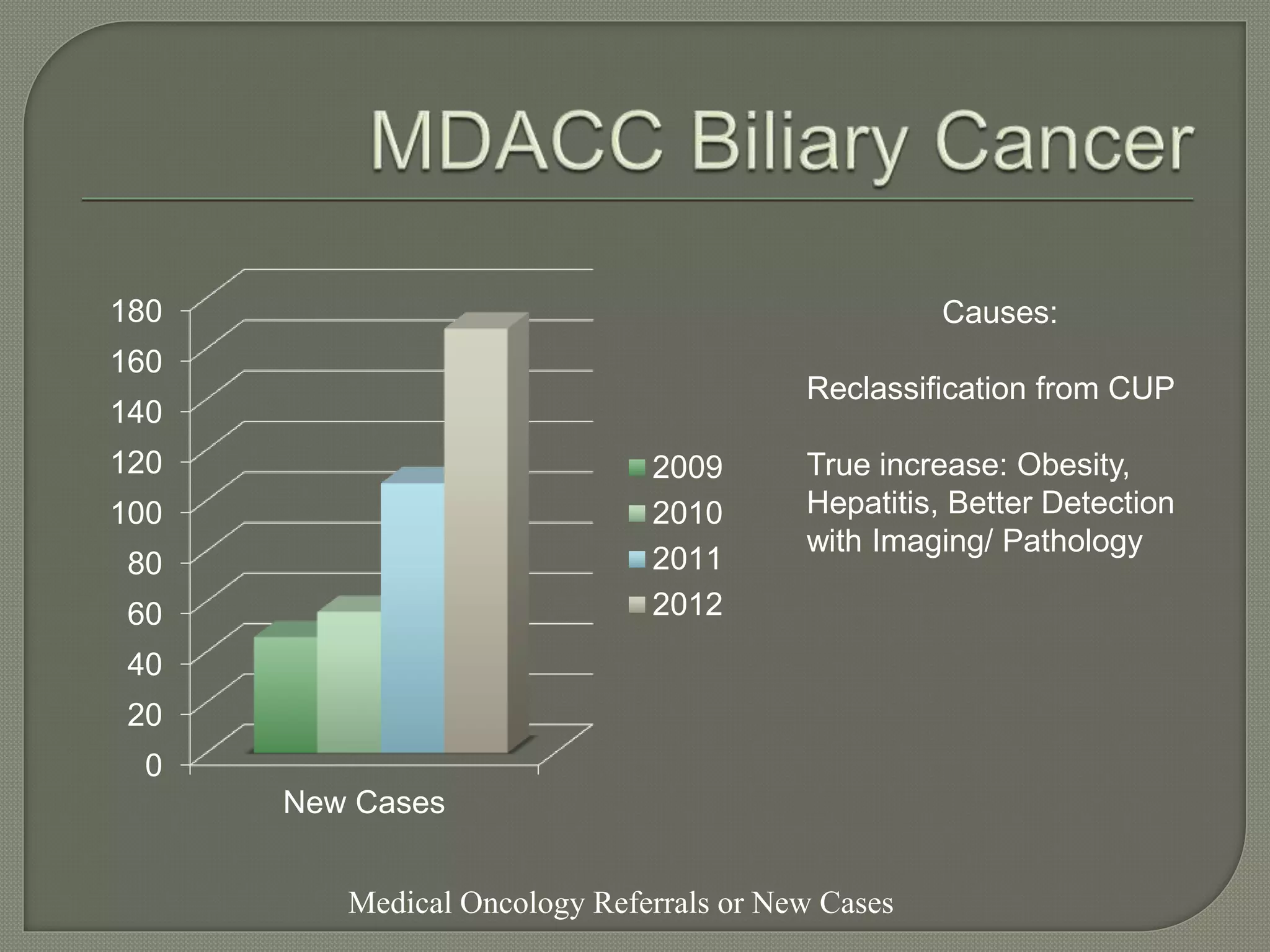
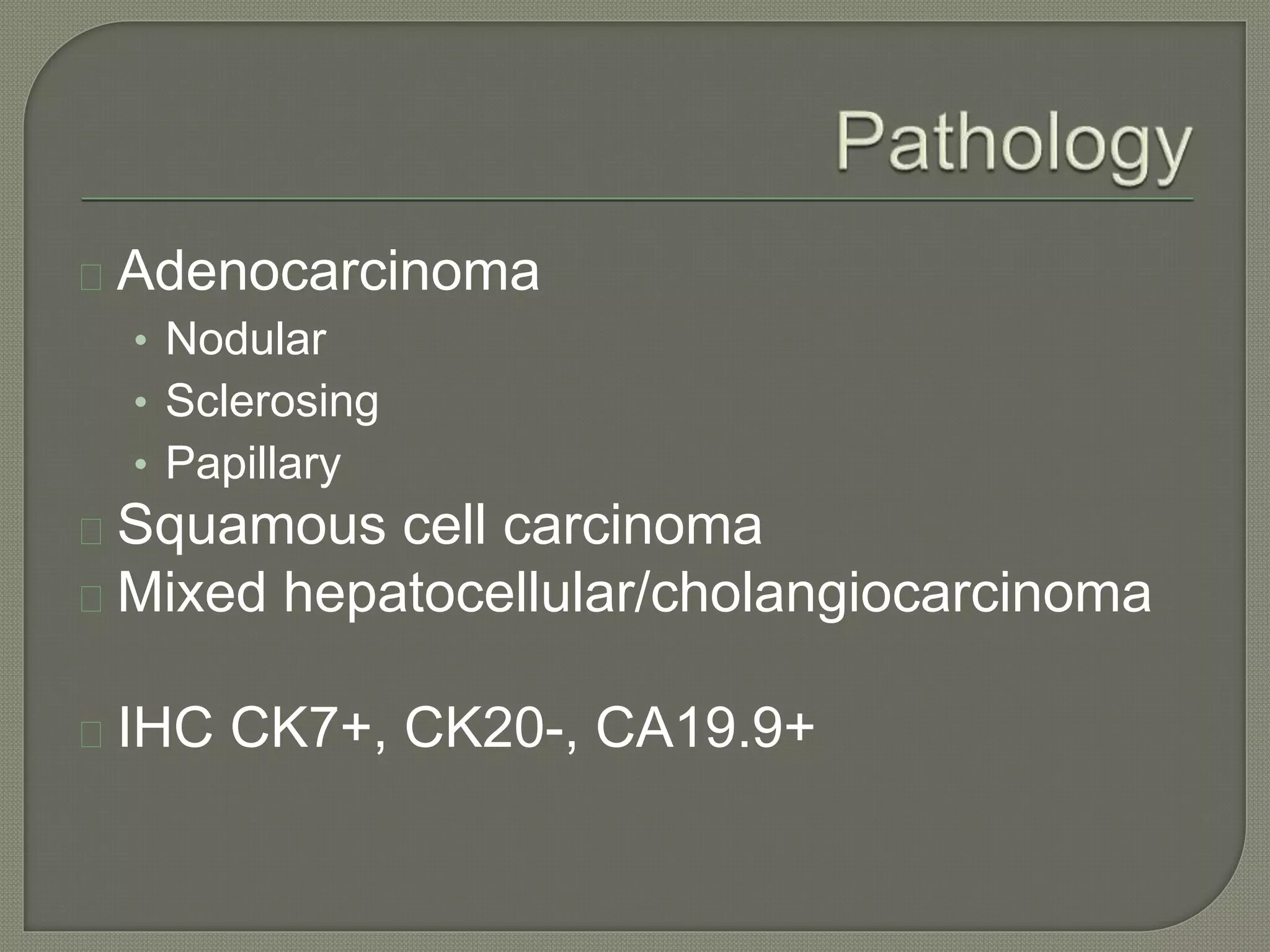
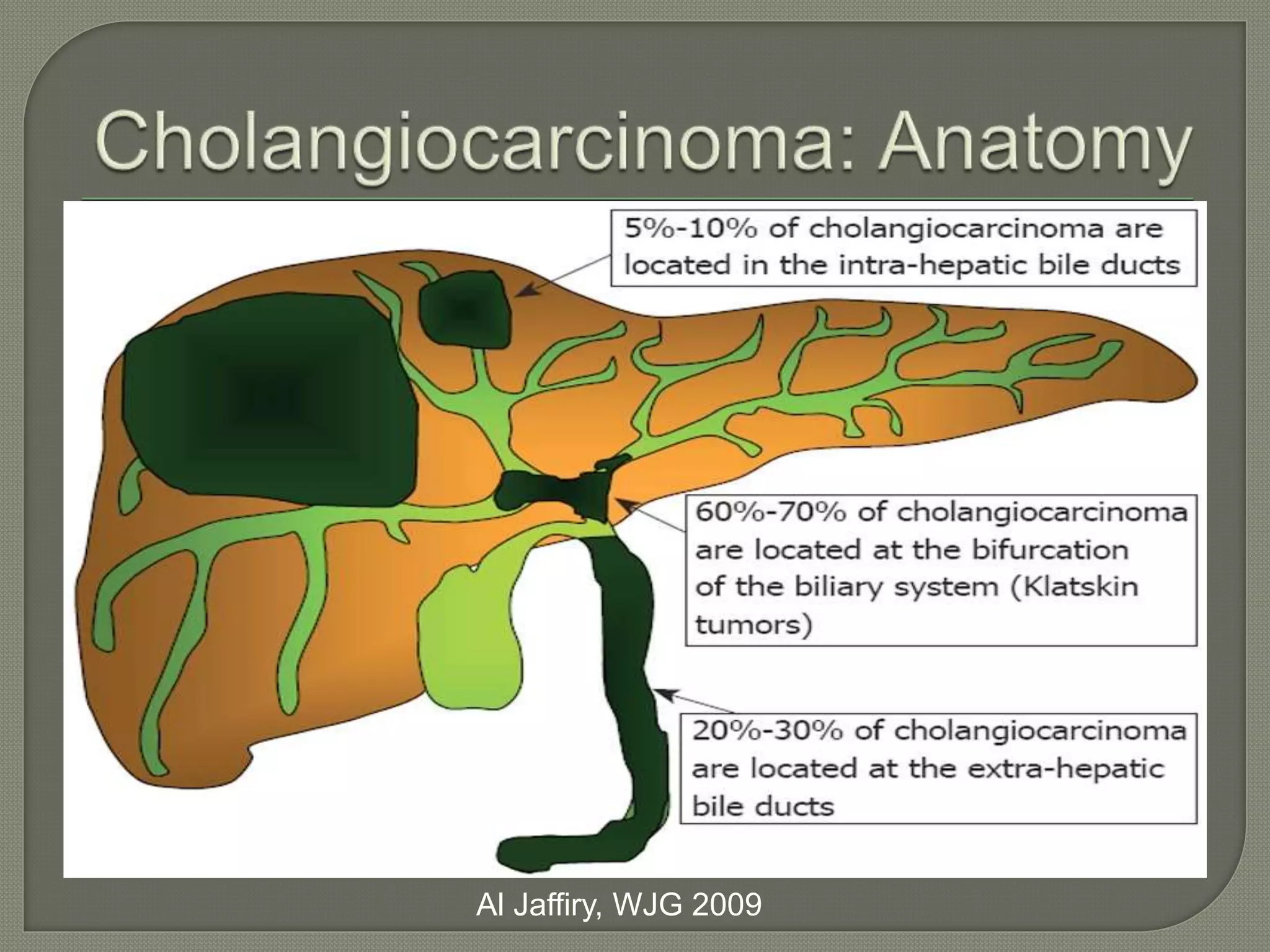
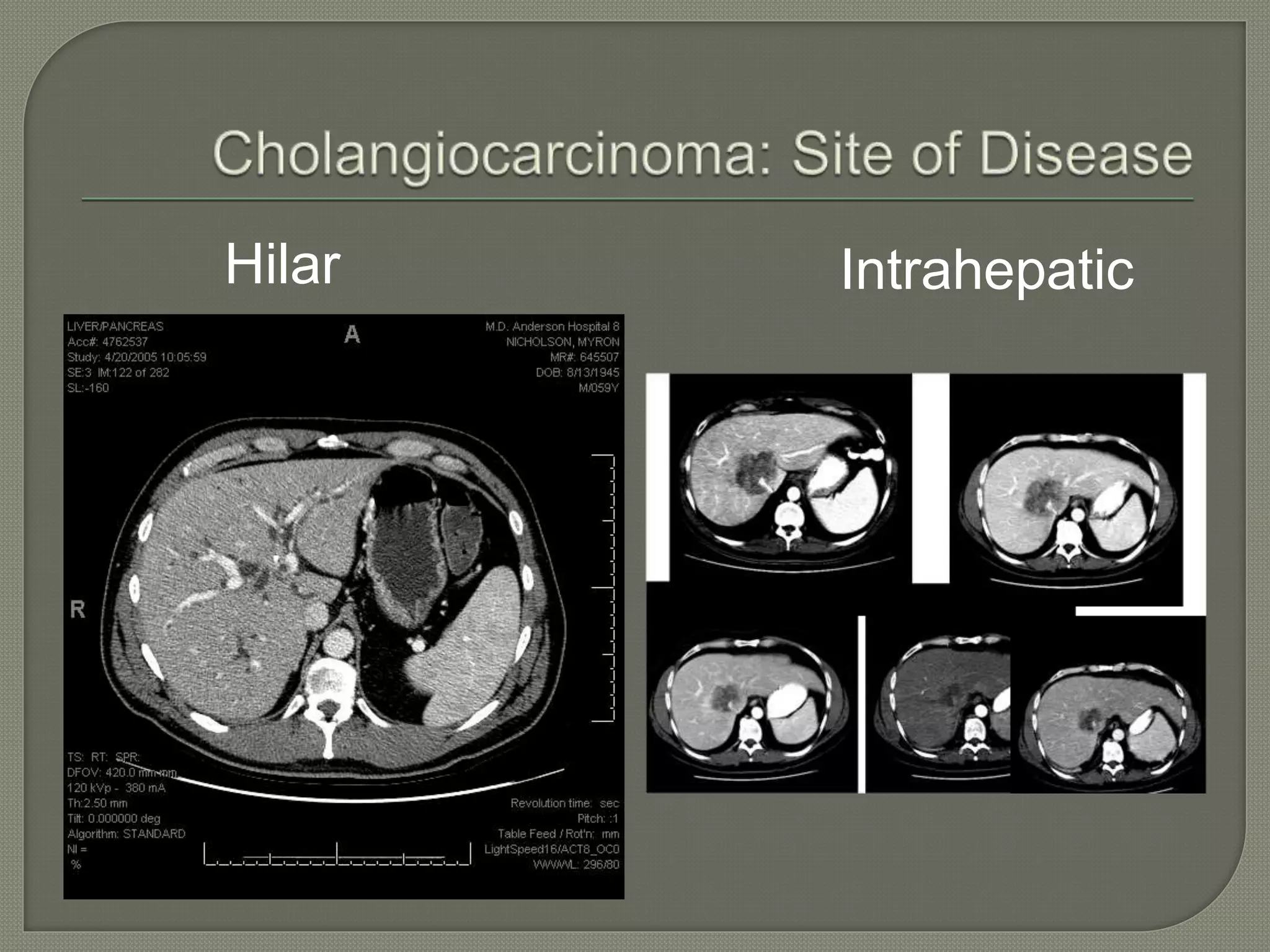
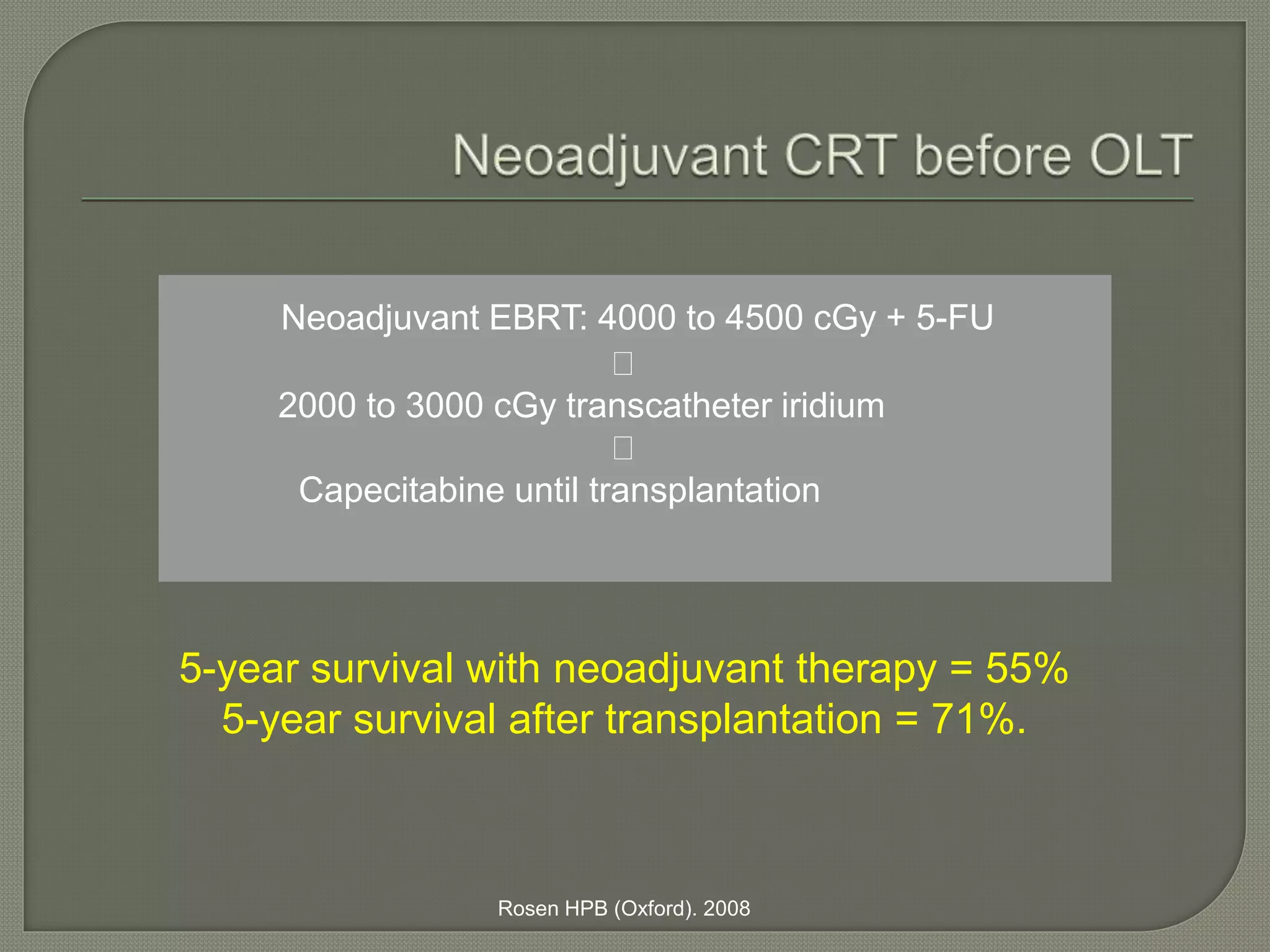
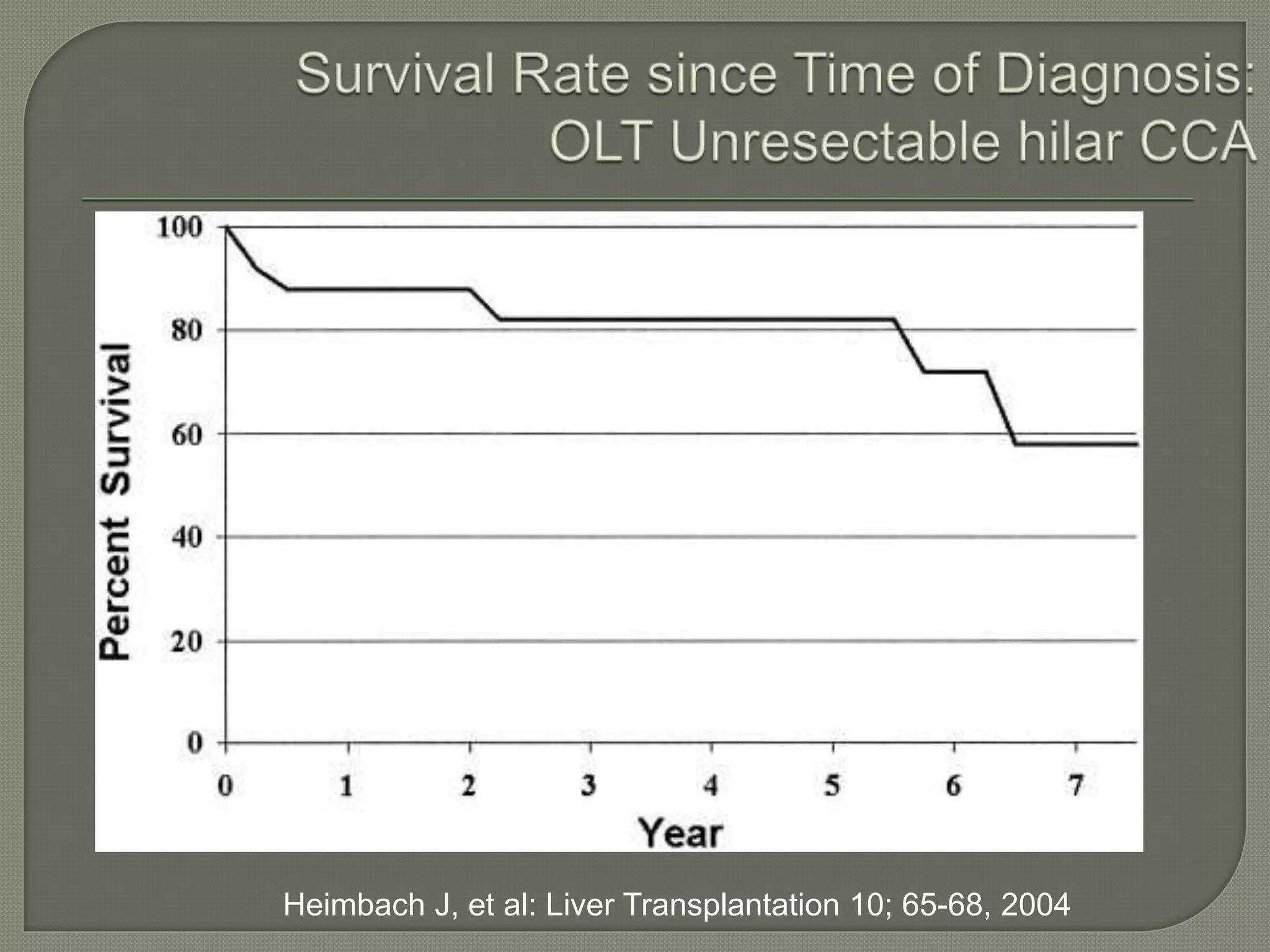
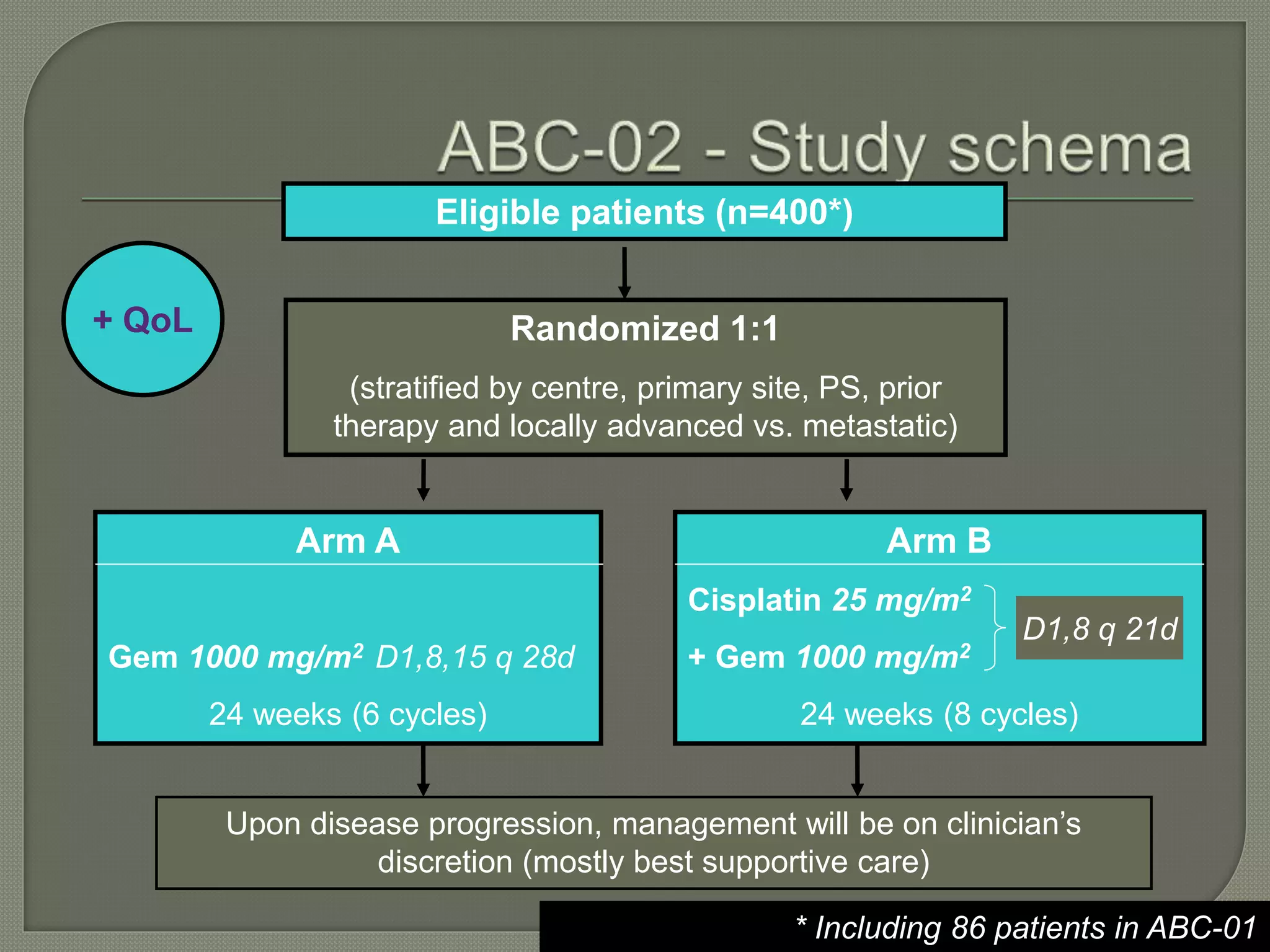
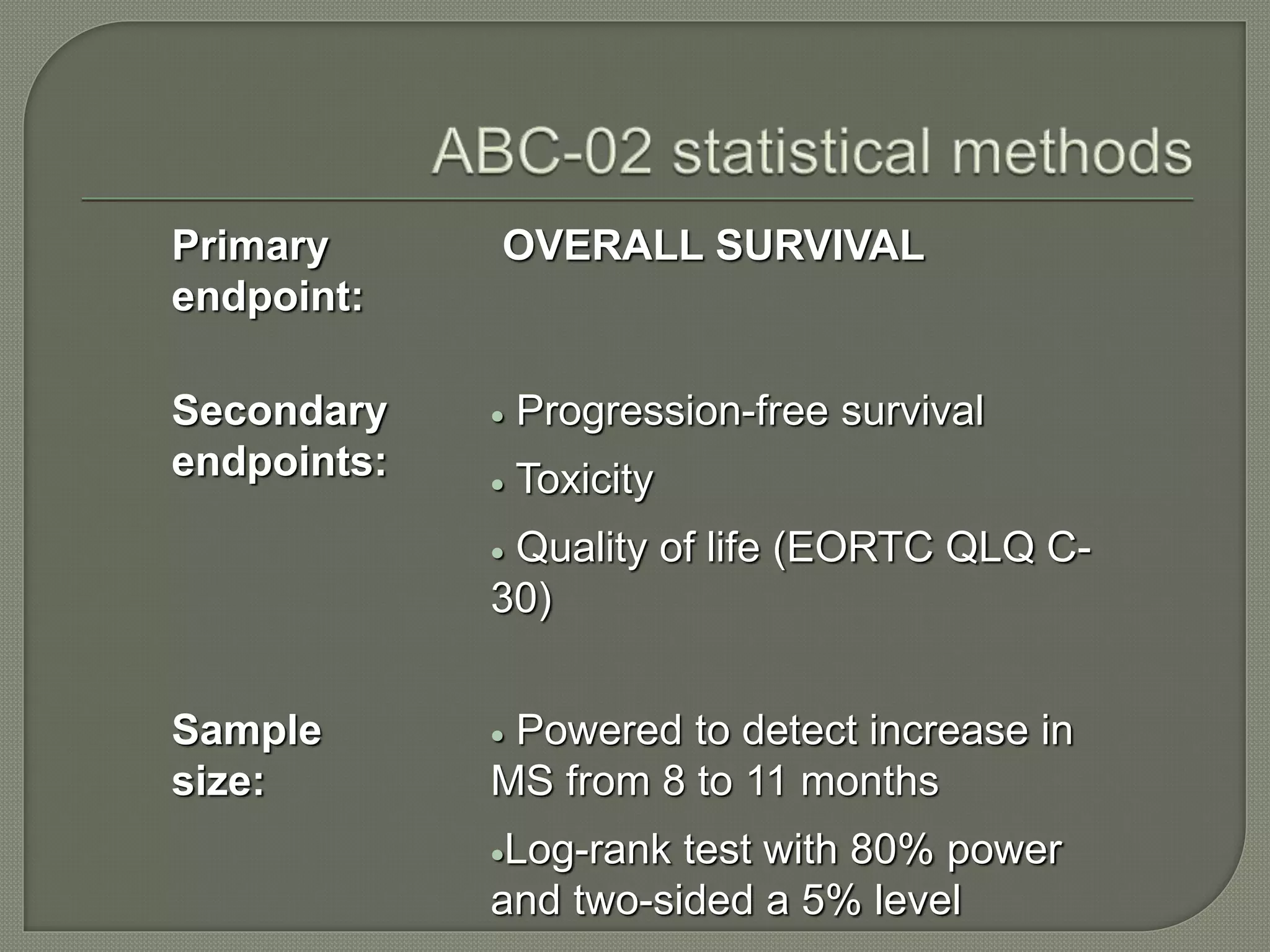
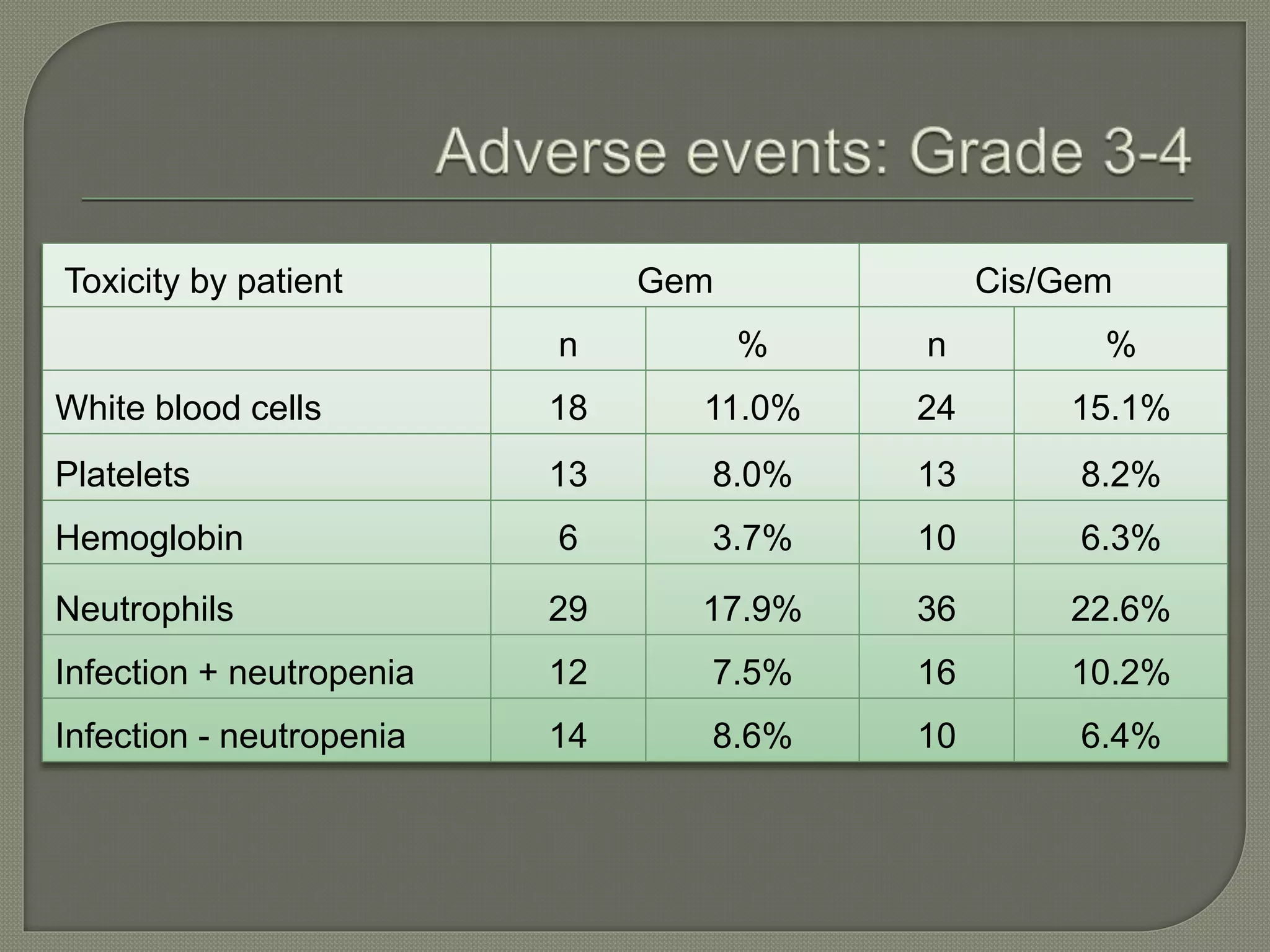
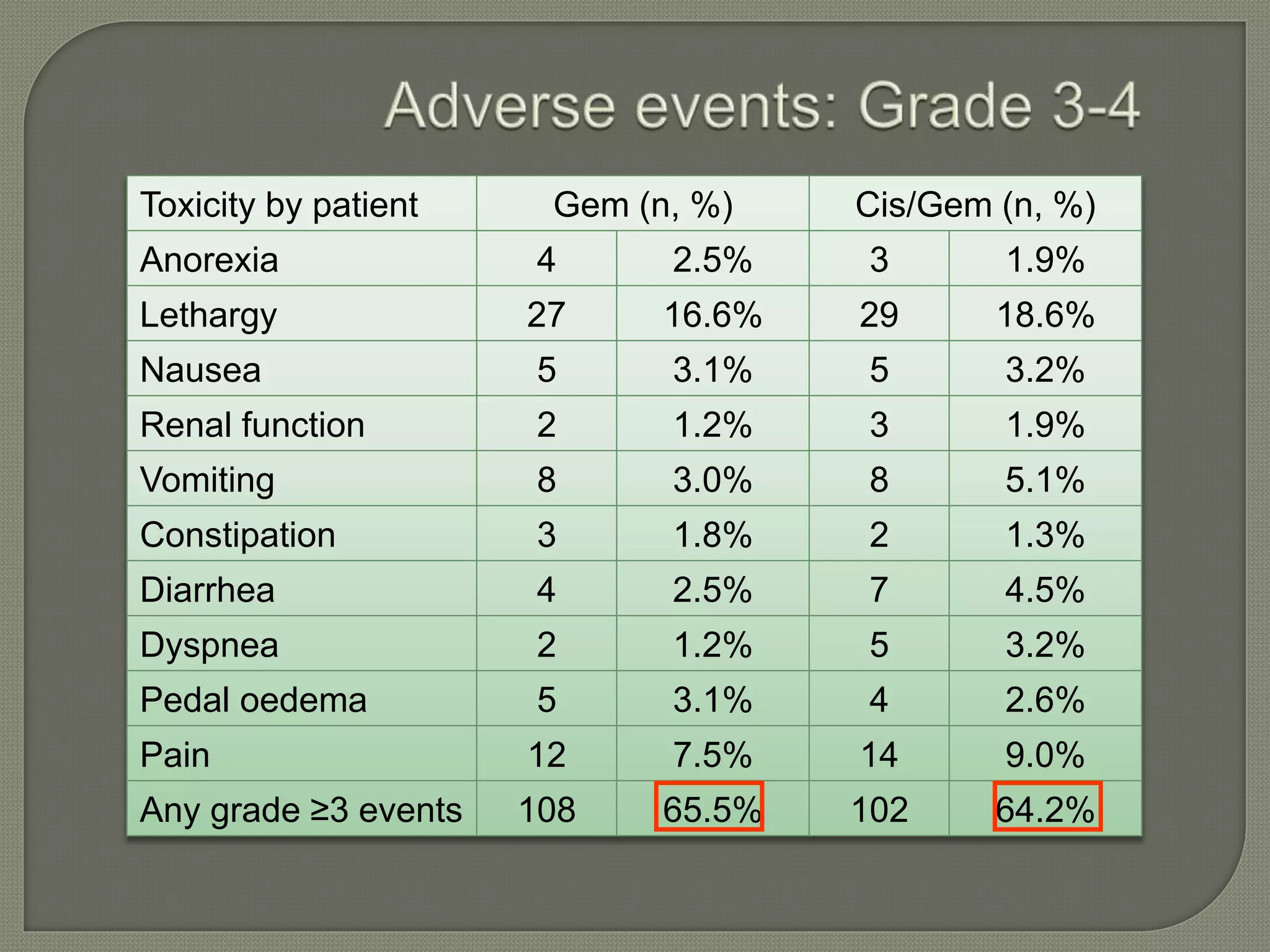
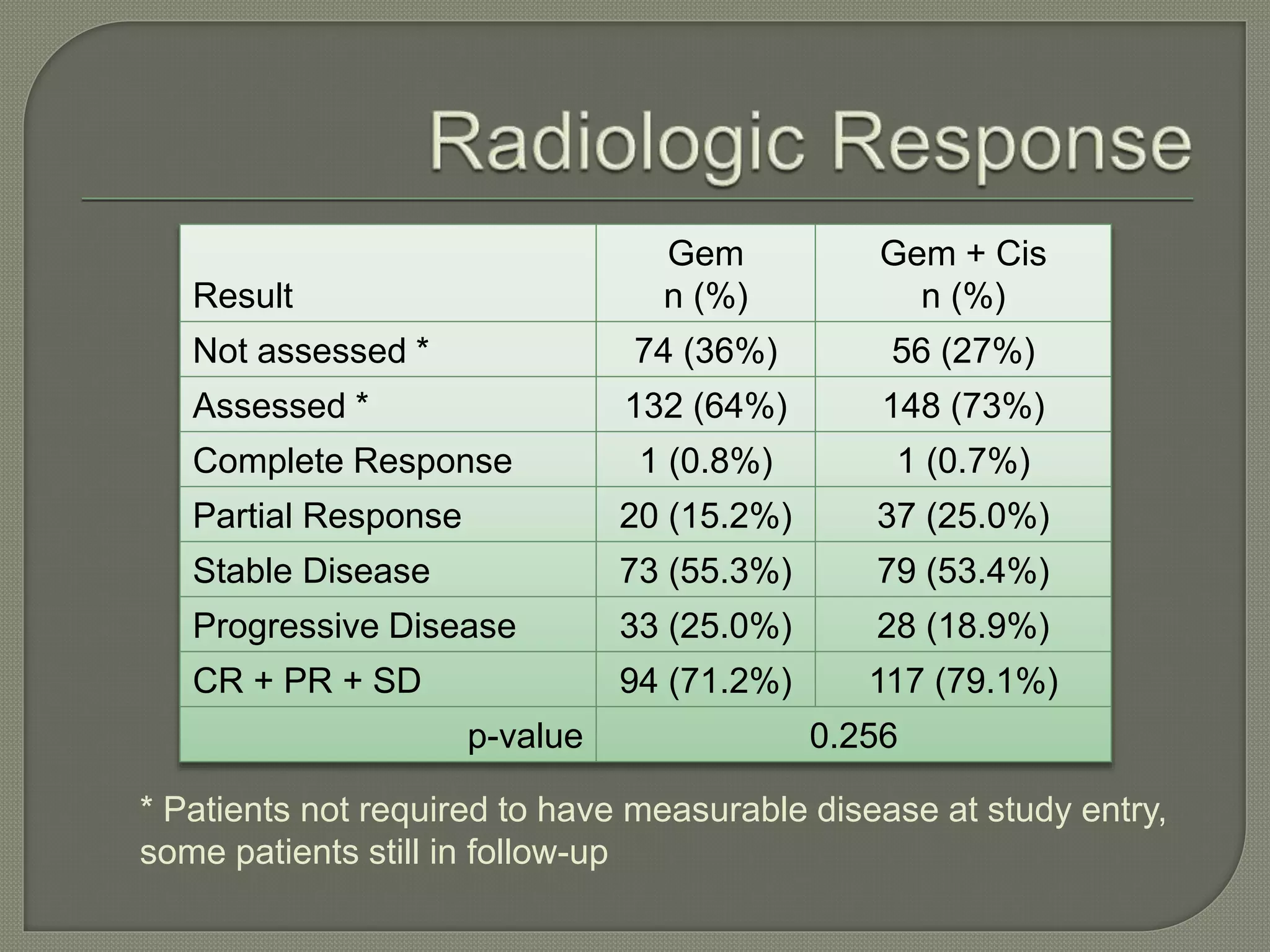
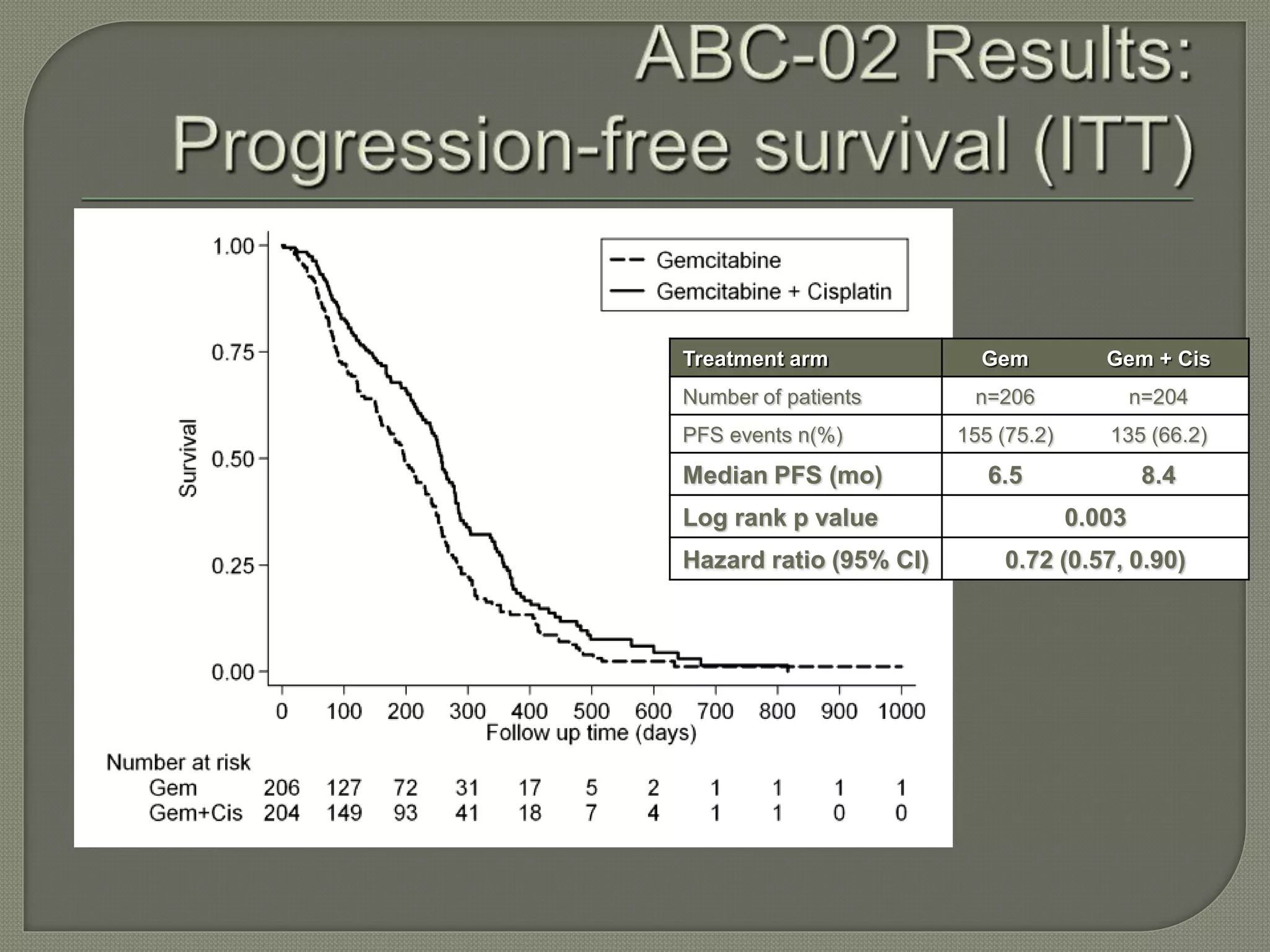
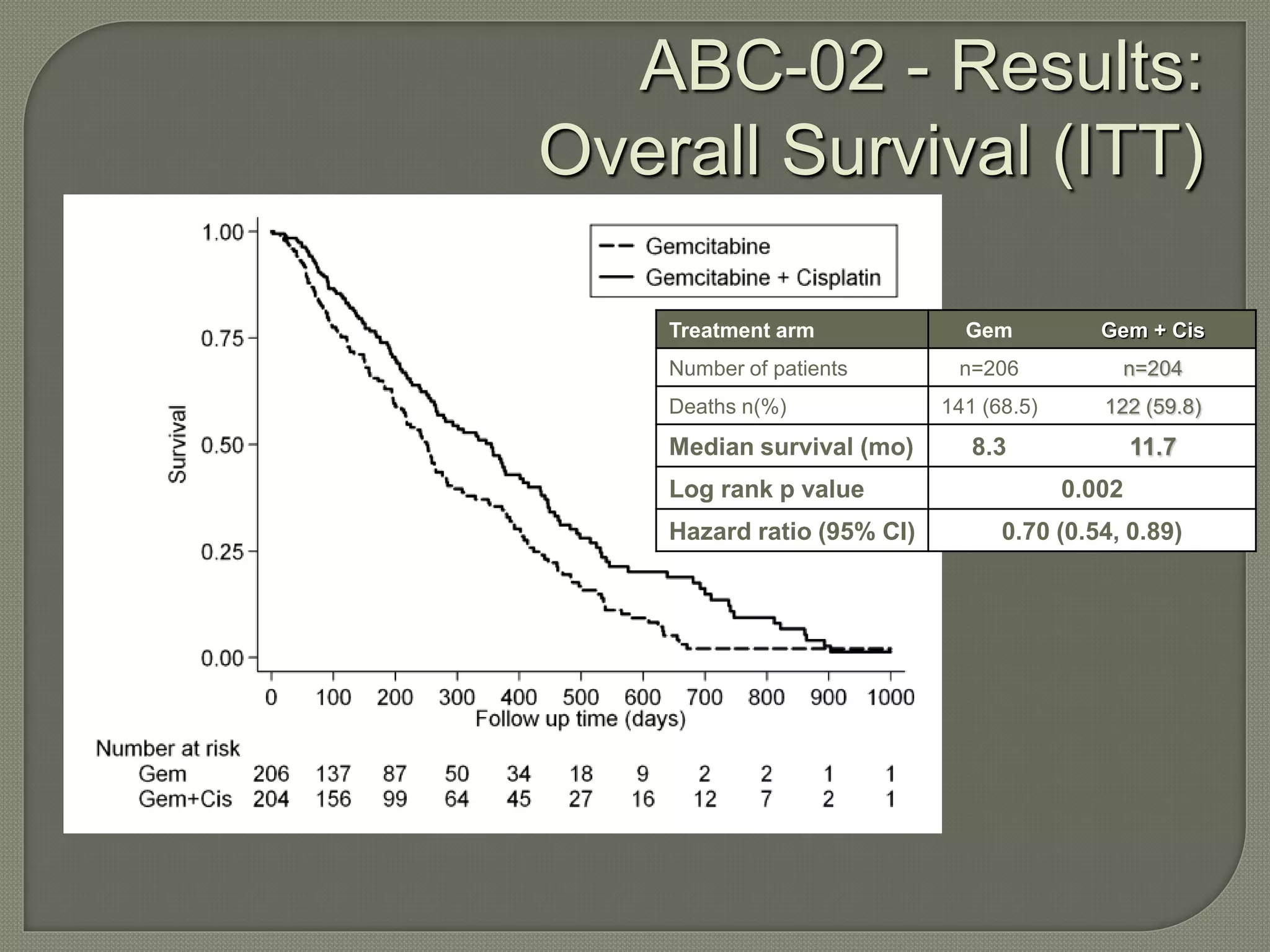
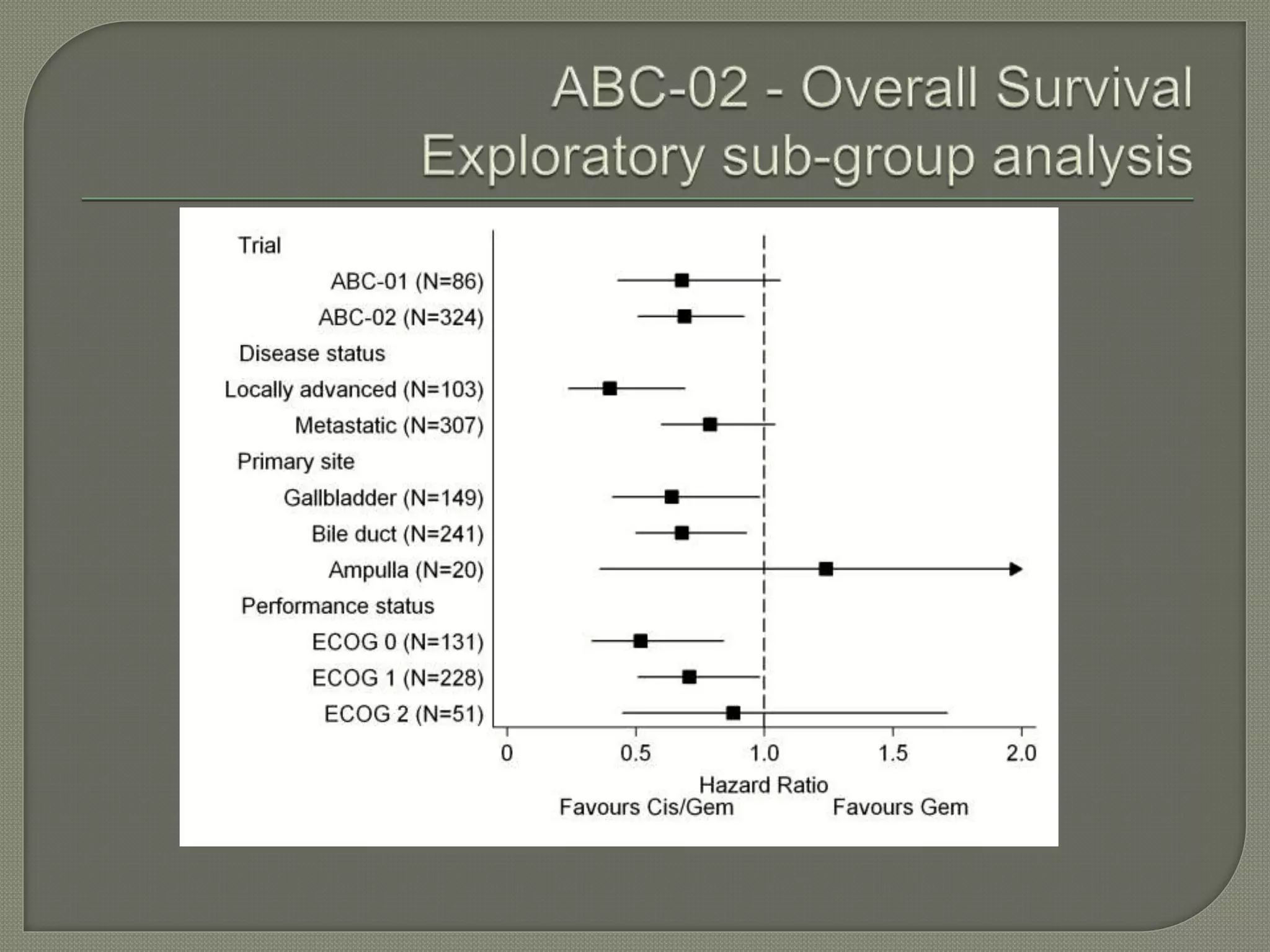
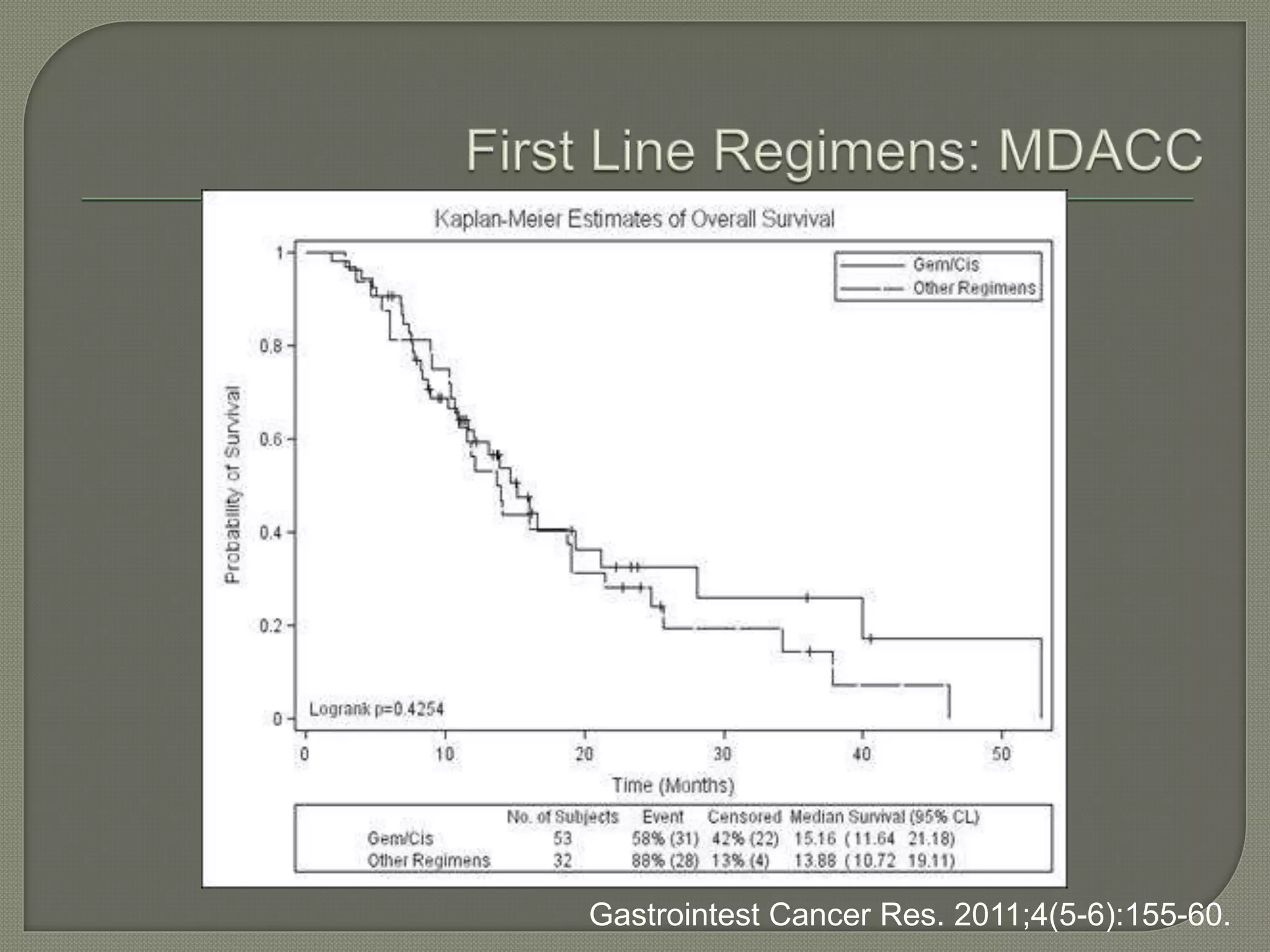
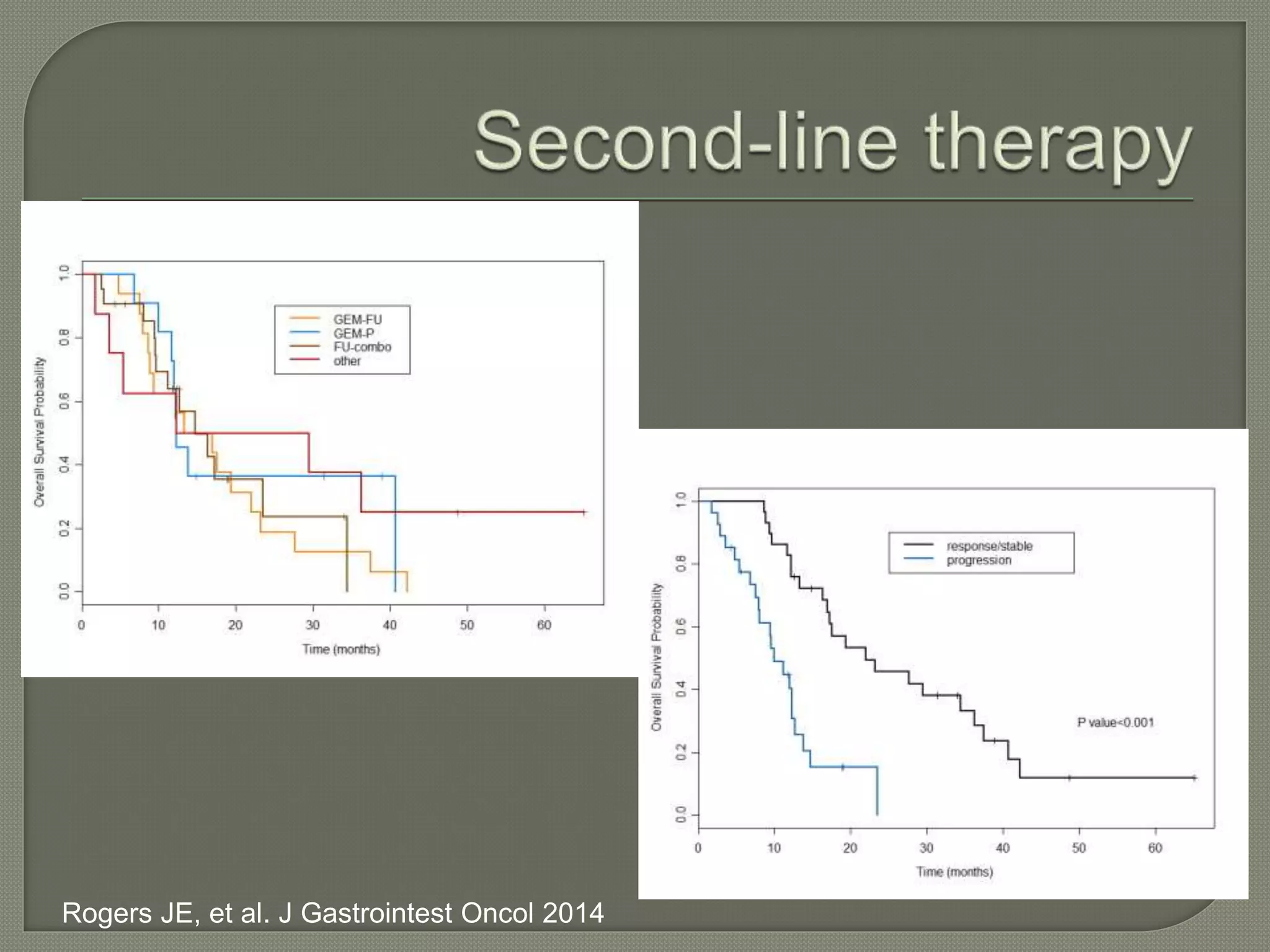
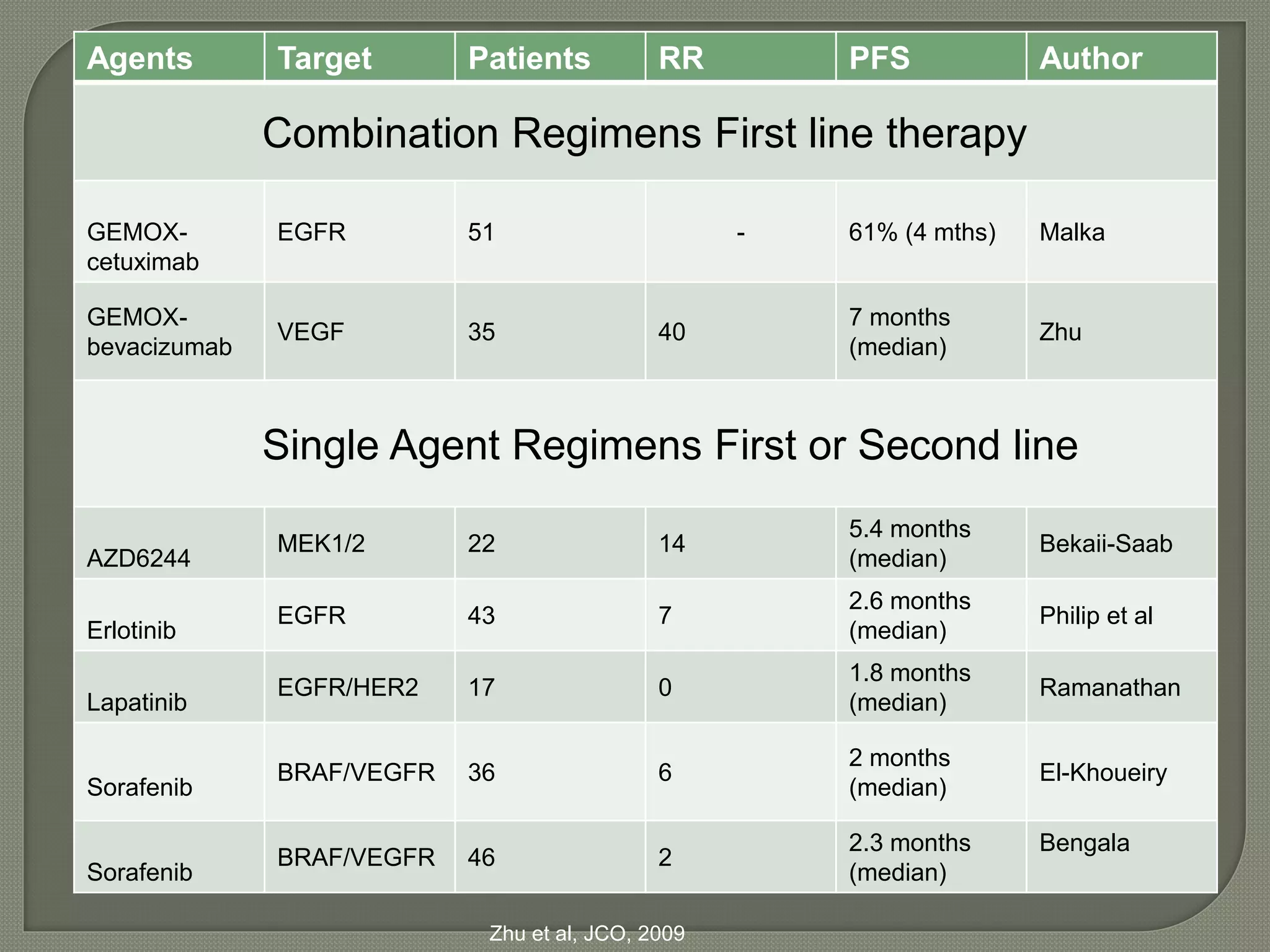
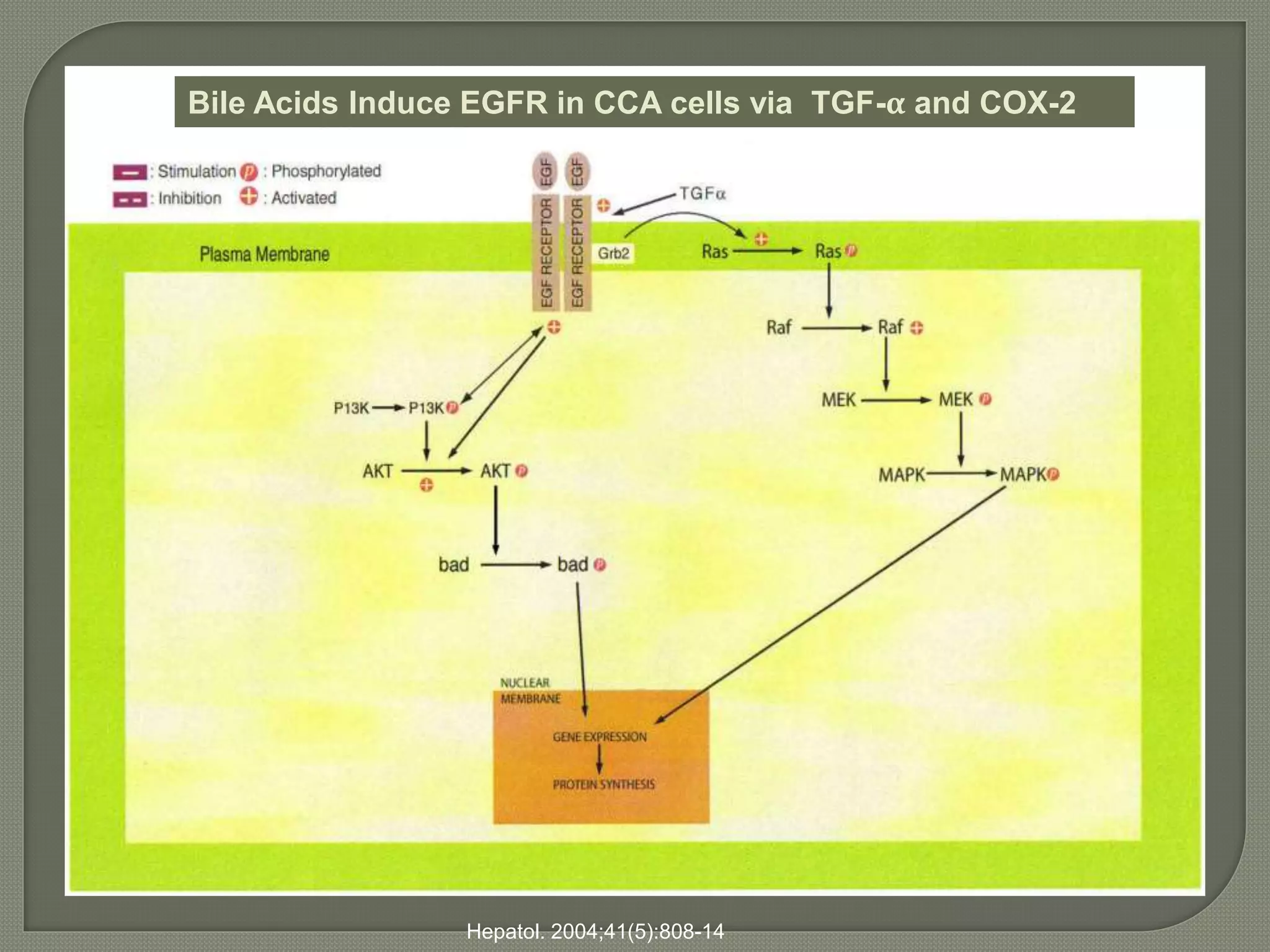
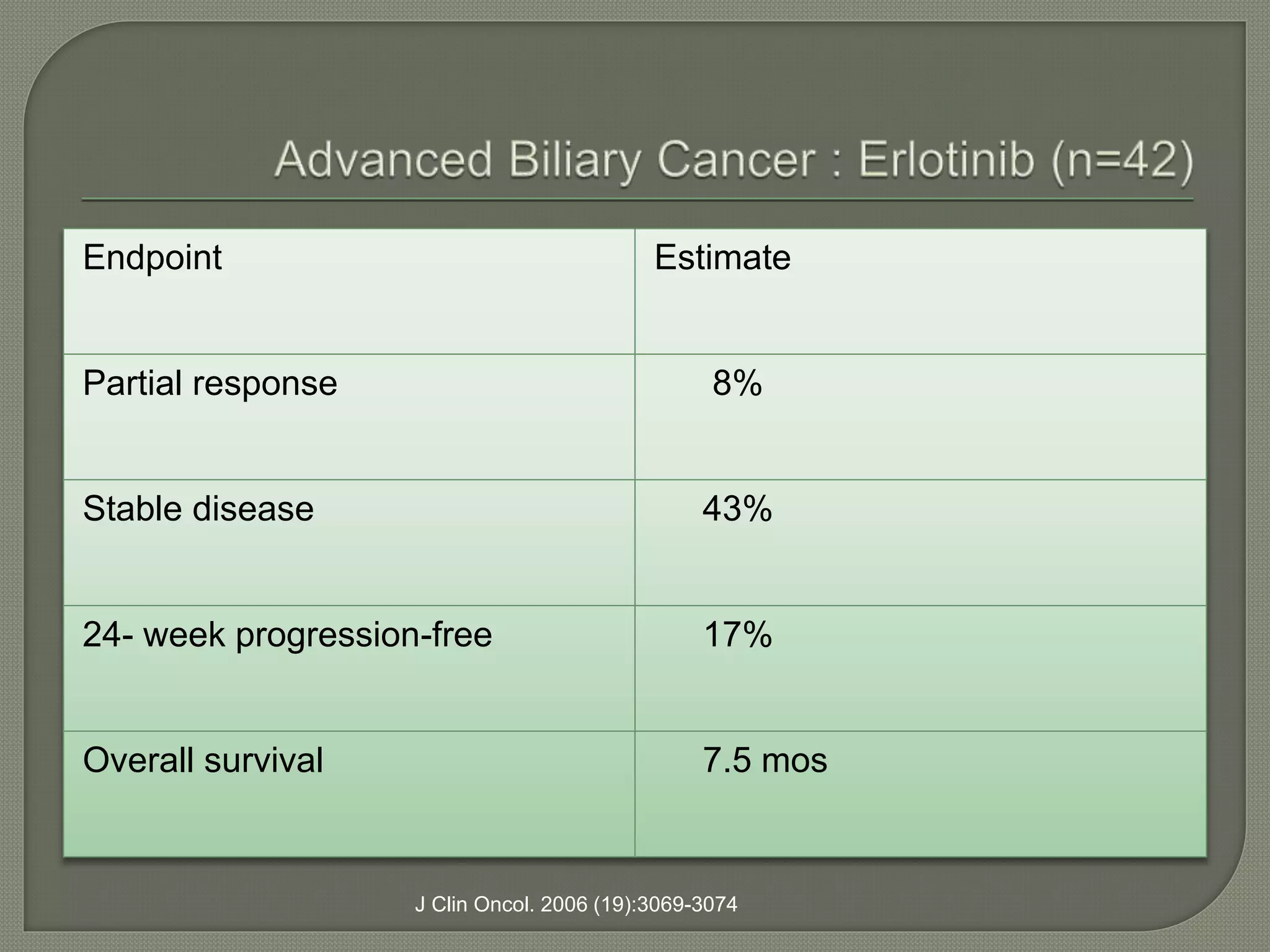
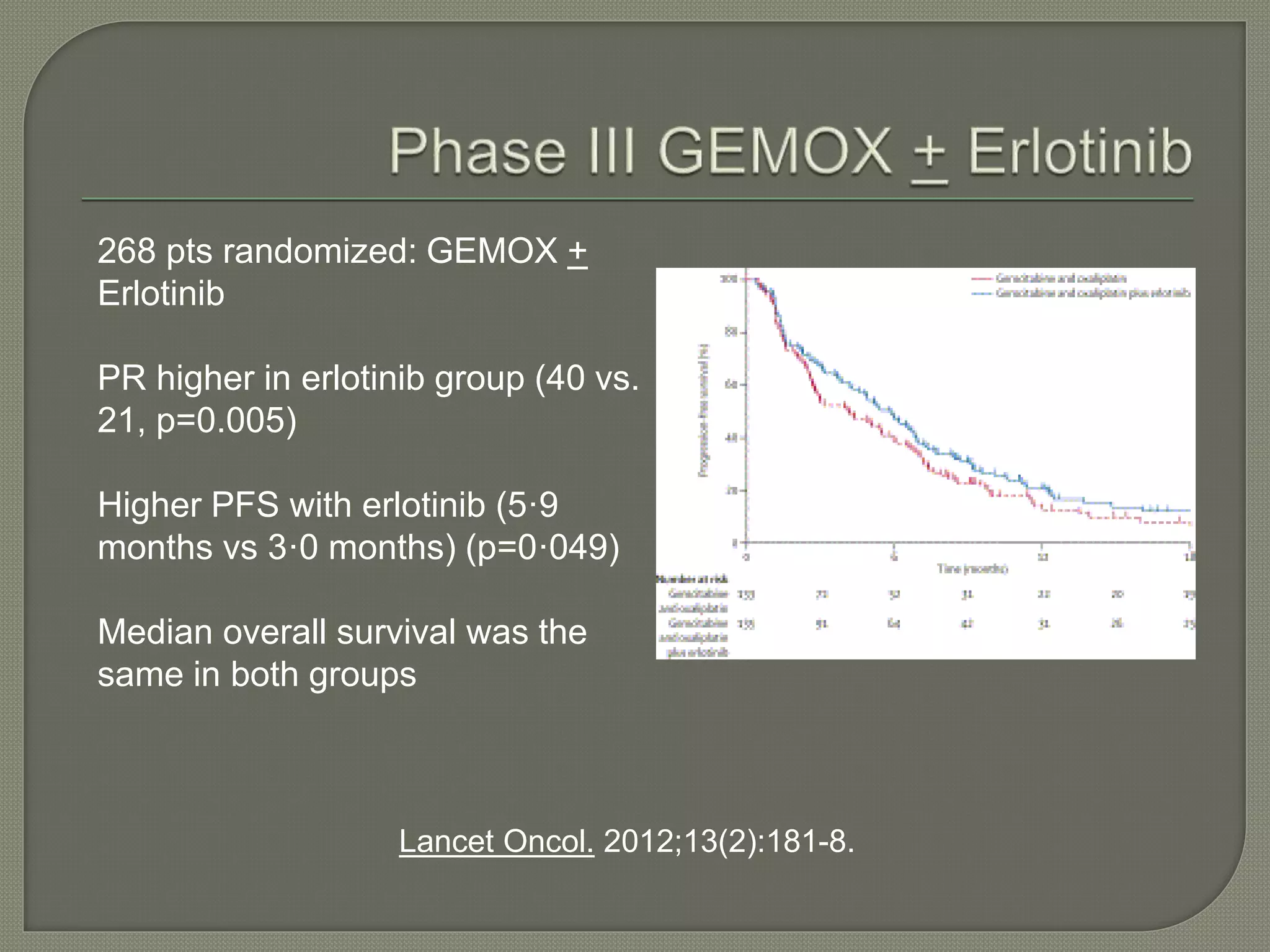
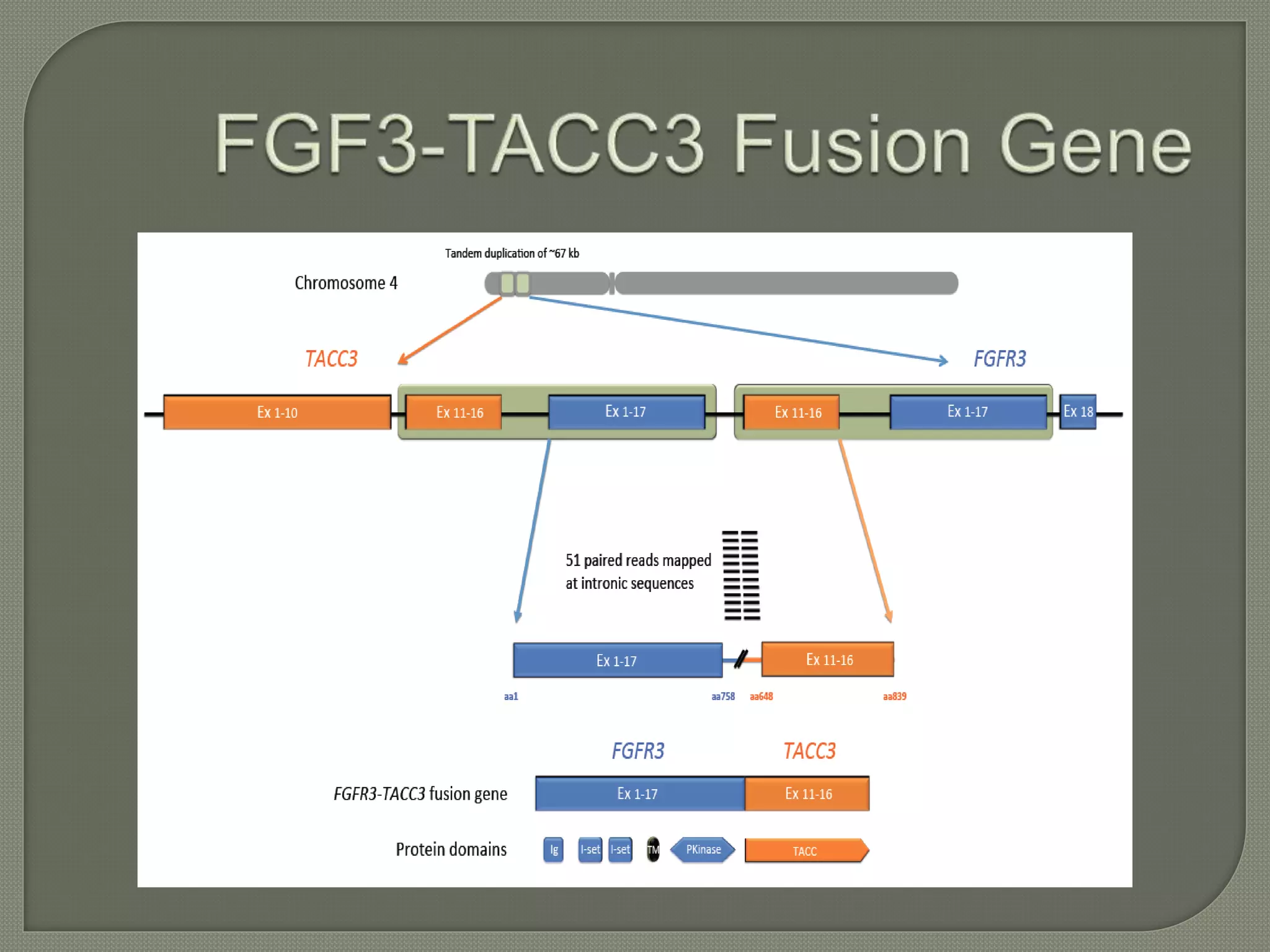
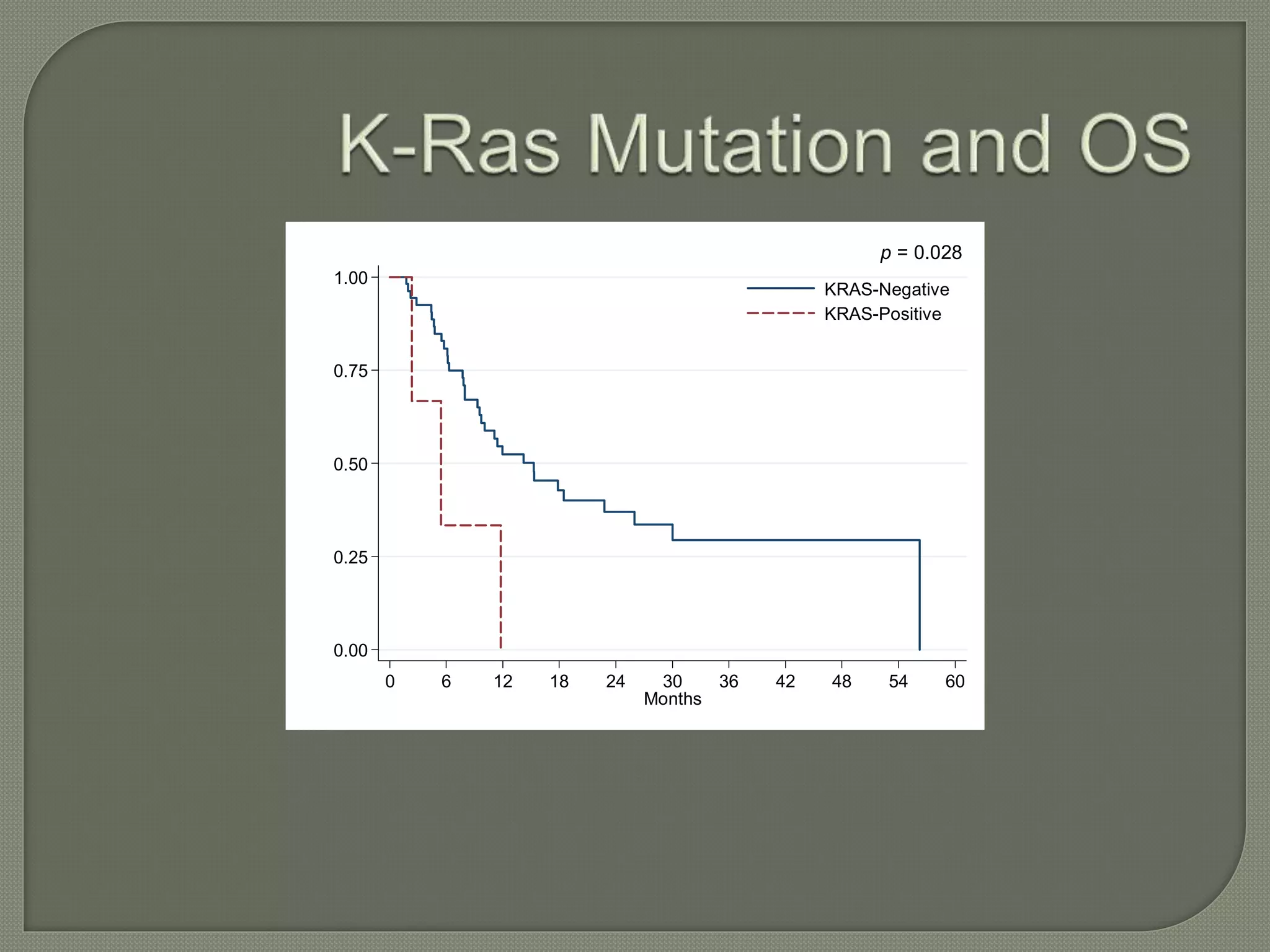
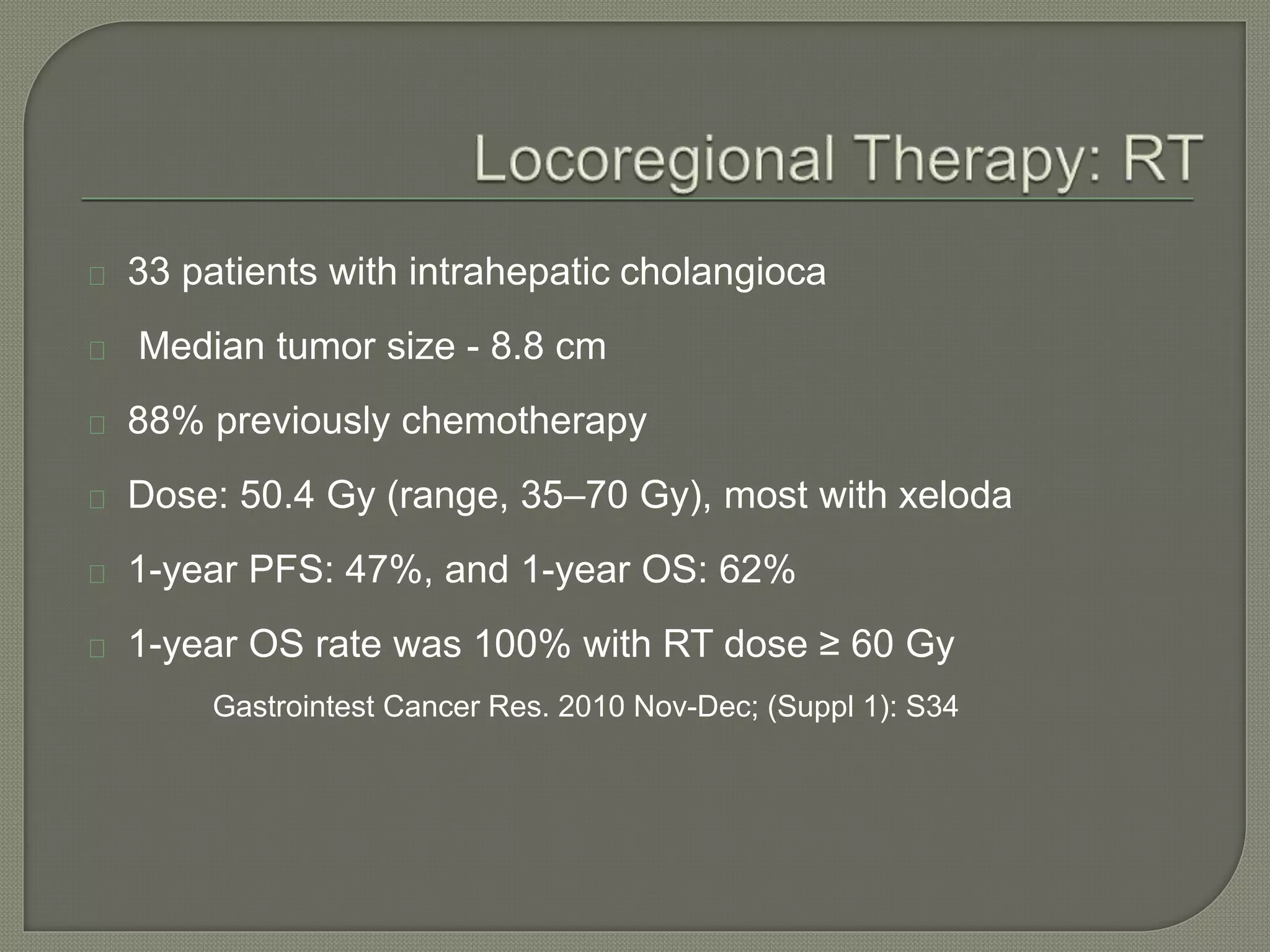
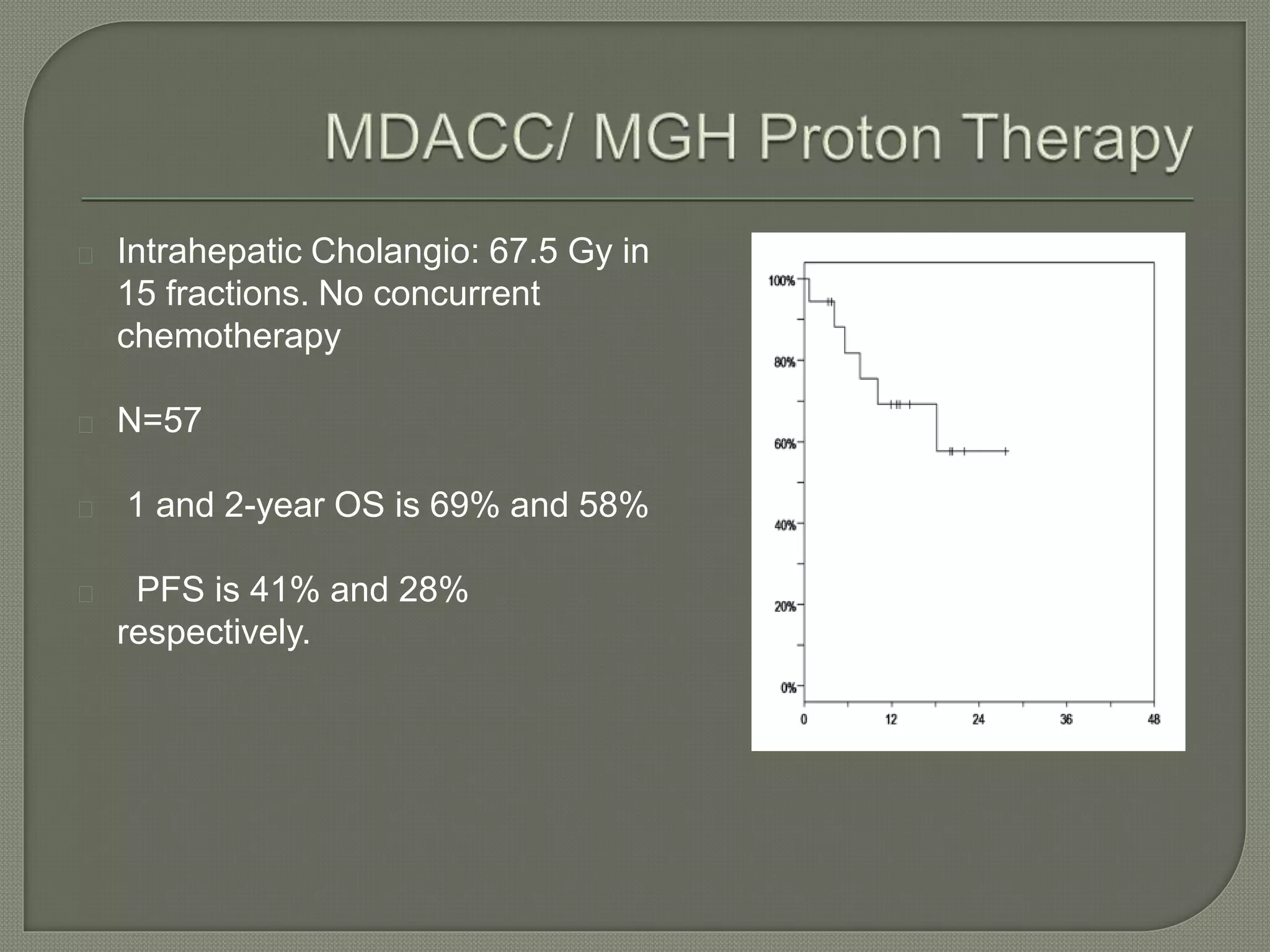
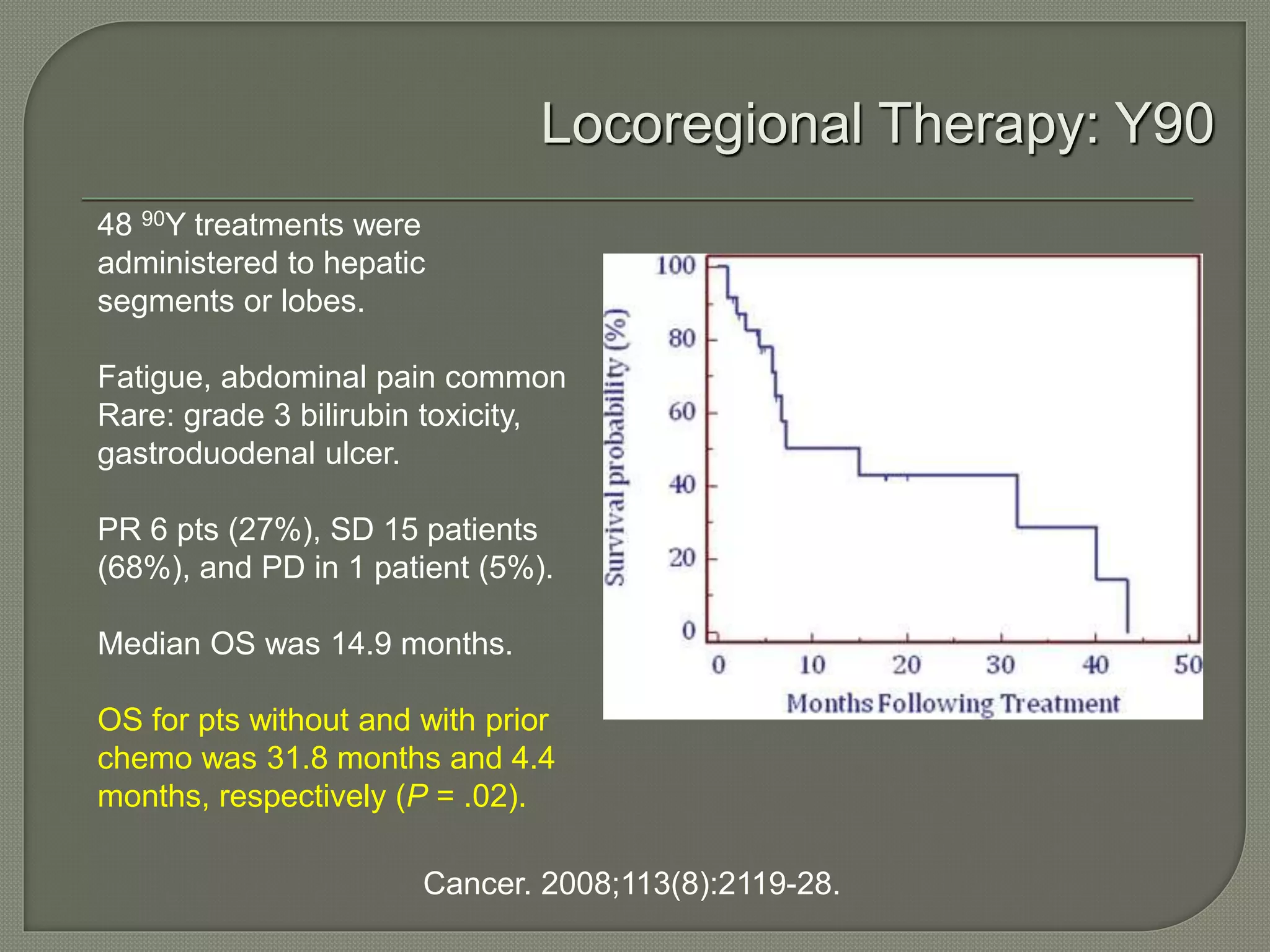
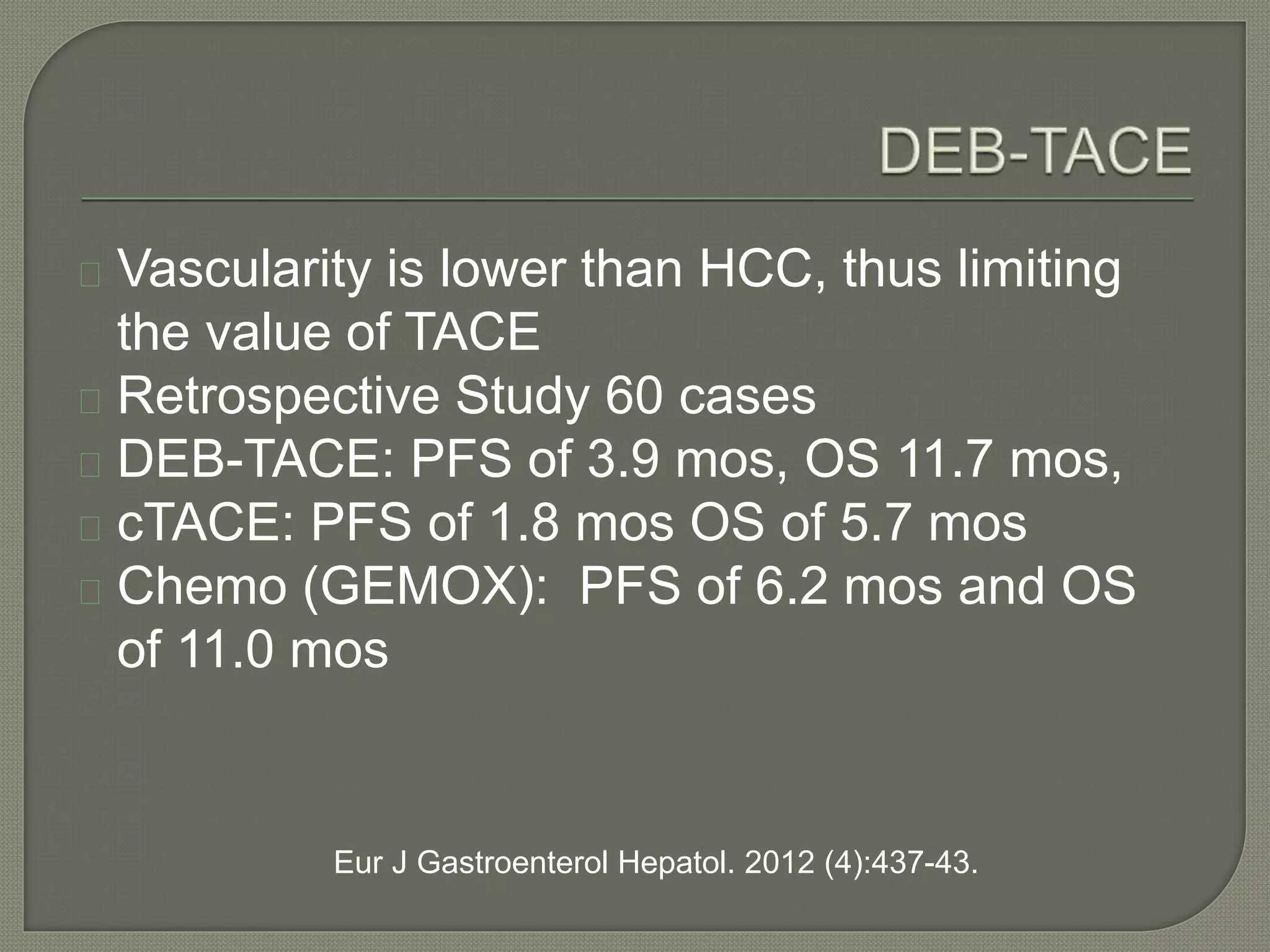
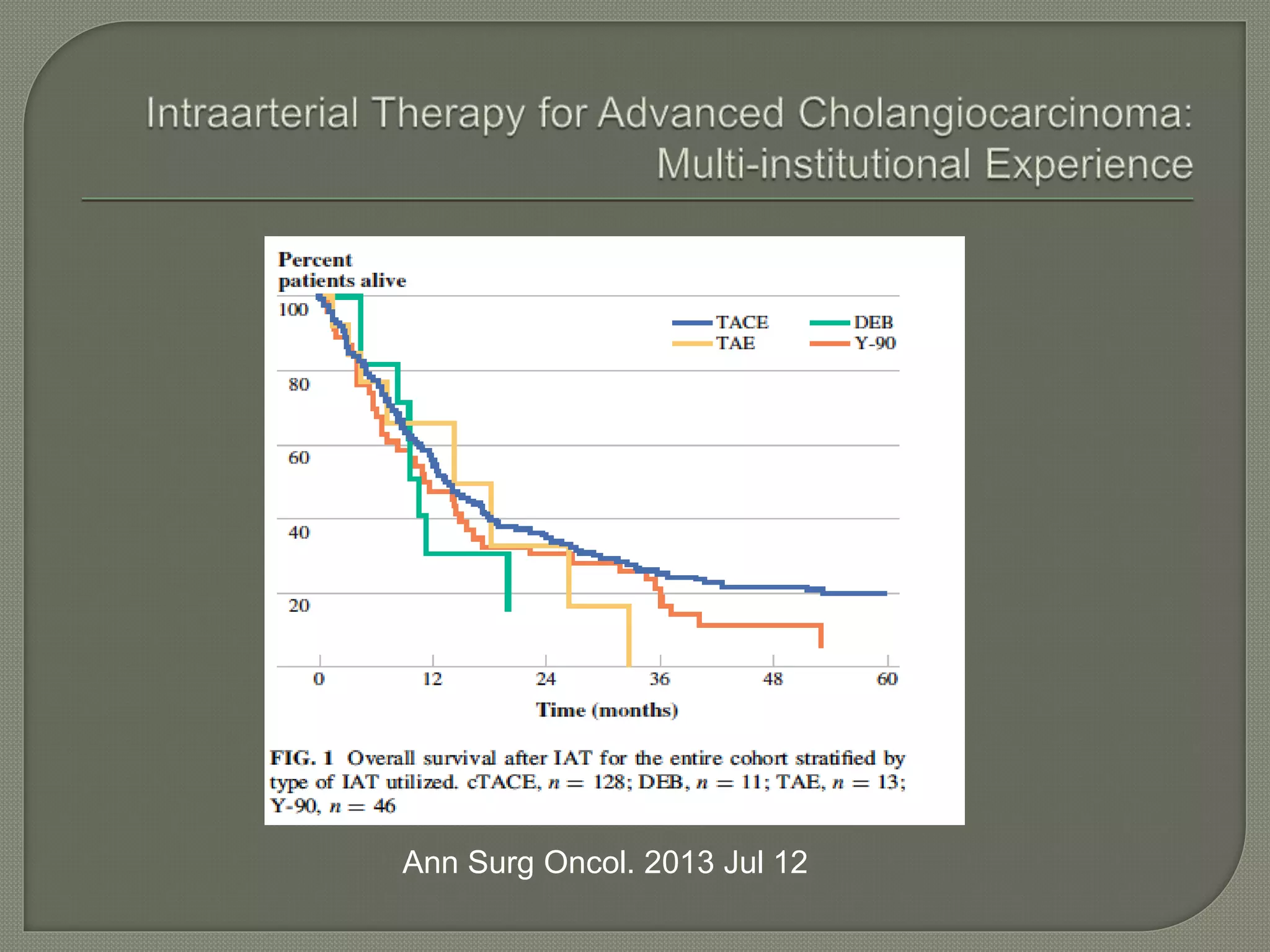
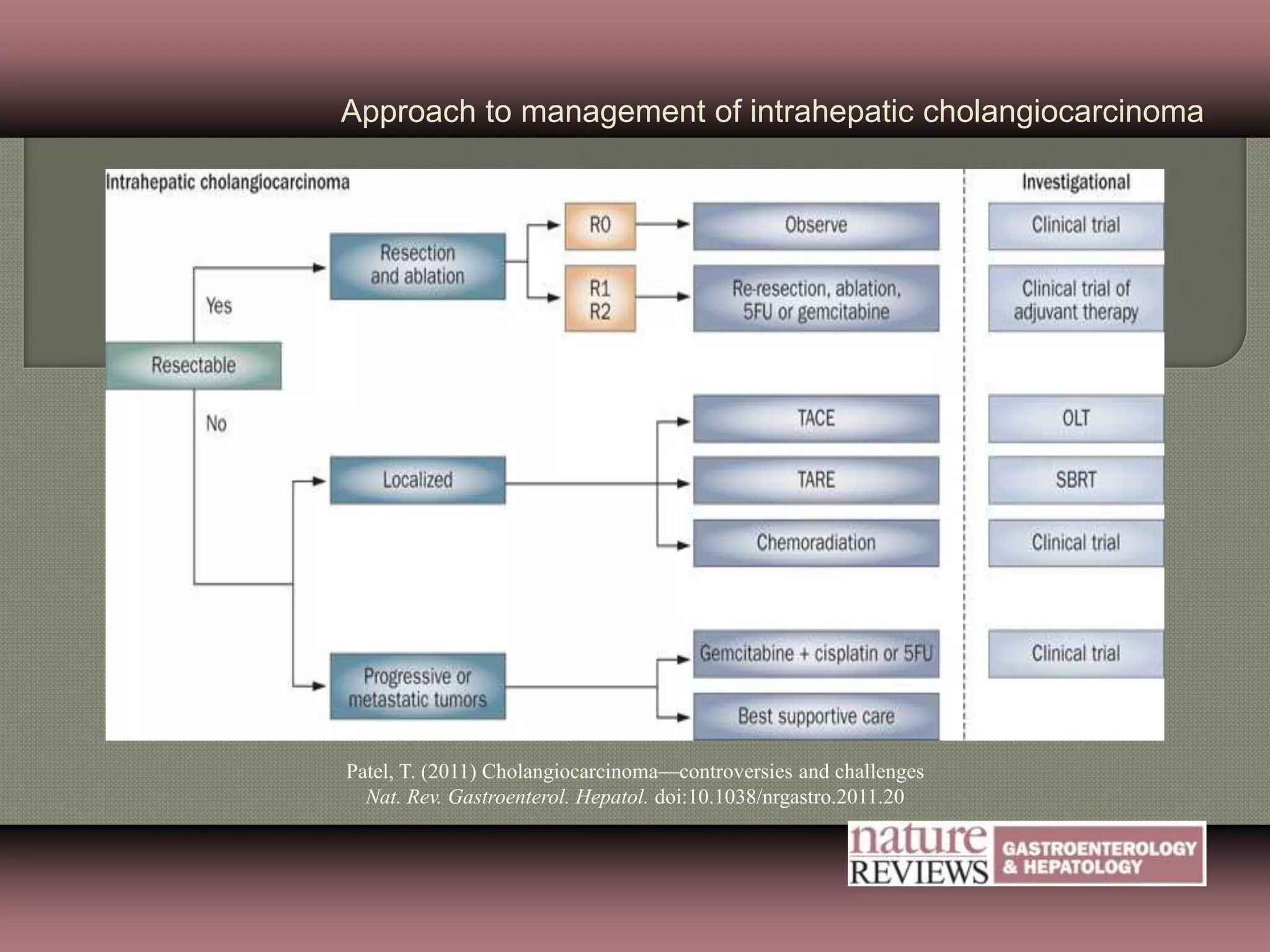
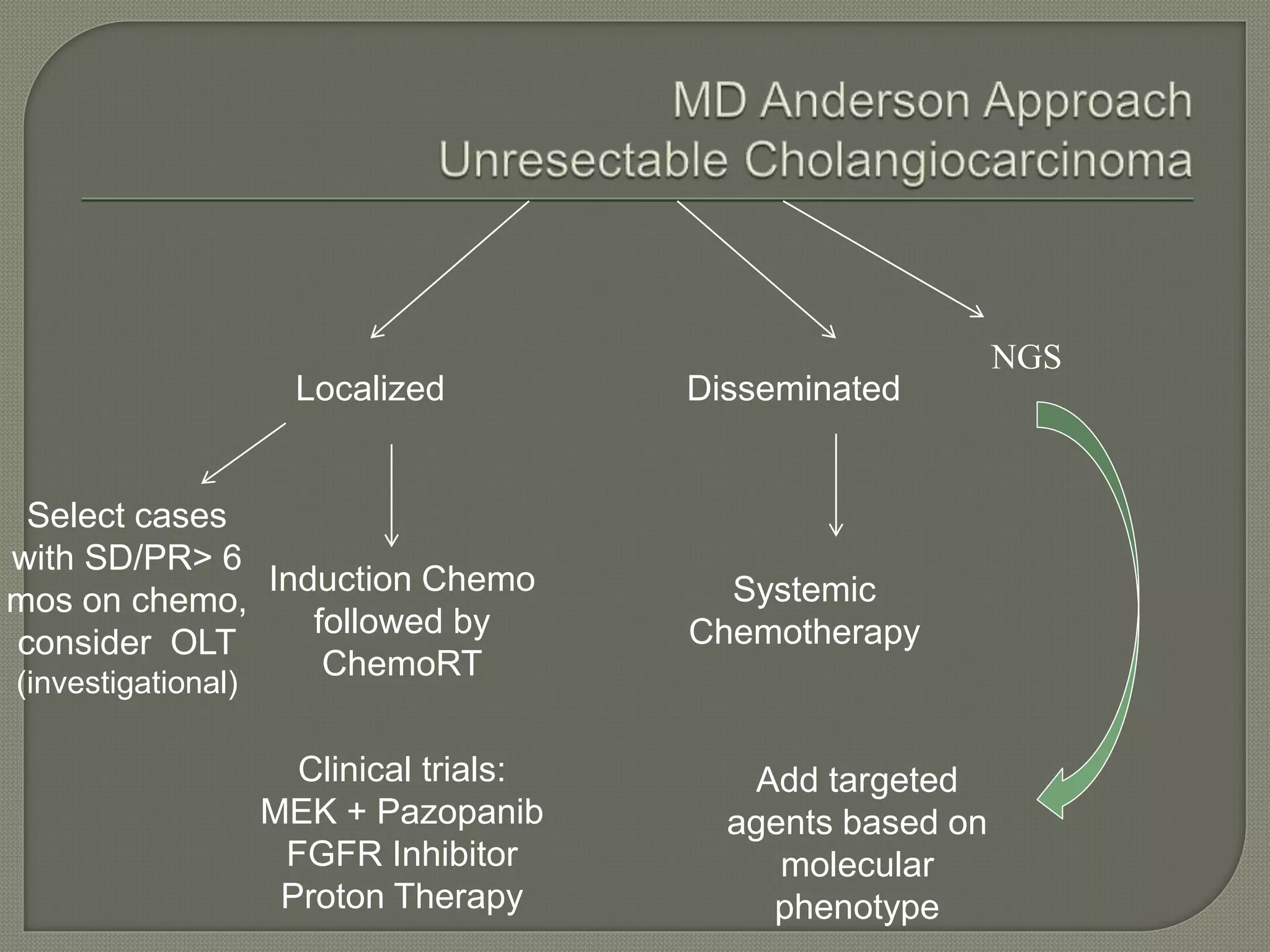
This document summarizes key points about the management of intrahepatic cholangiocarcinoma. It finds that surgical resection provides the best chance for long-term survival, with 5-year survival rates of 20-30% for resectable disease. For unresectable tumors, options include liver transplantation in select patients and local therapies like radiofrequency ablation, transarterial chemoembolization, and yttrium-90 microsphere treatment, which have shown some promise for improving survival. Systemic chemotherapy with gemcitabine and cisplatin is the standard first-line treatment based on improved survival seen in a phase III trial, while various targeted agents in combination with chemotherapy are under investigation in clinical trials