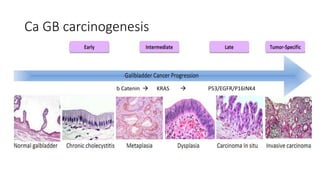
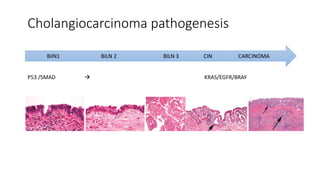
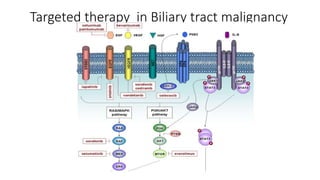
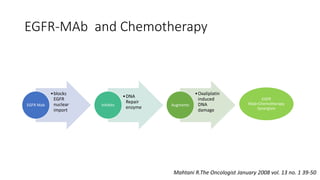
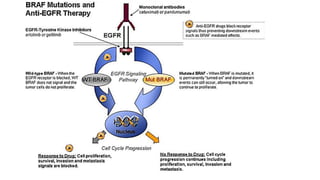
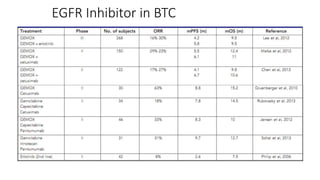
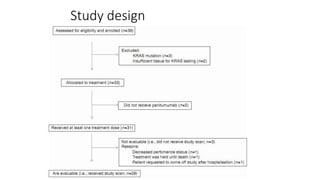
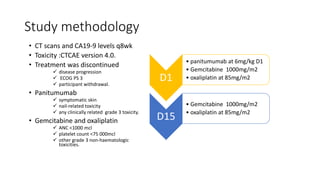
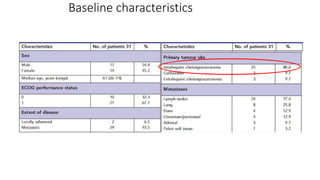
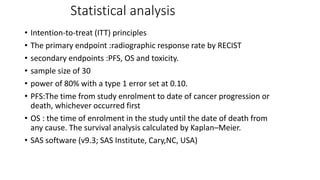
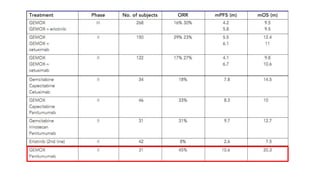
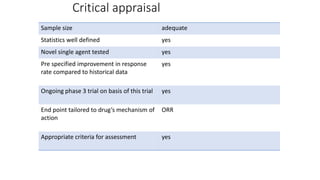
1) This open-label phase II trial evaluated the efficacy of panitumumab in combination with gemcitabine and oxaliplatin (GemOx) in patients with KRAS wild-type biliary tract cancer.
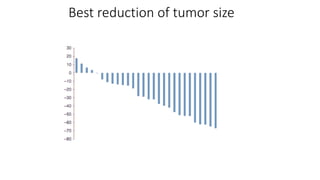
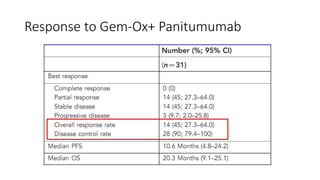
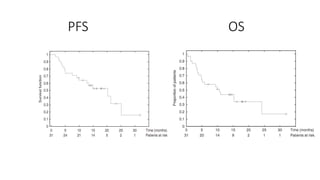
2) The response rate was 50% with this combination, and median progression-free survival and overall survival were 10.6 months and 20.3 months respectively.
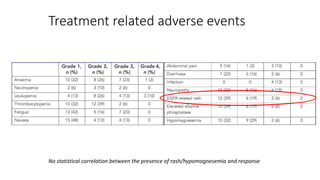
3) The combination showed manageable toxicity and was well-tolerated by patients.