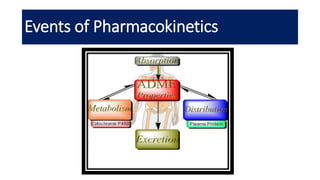
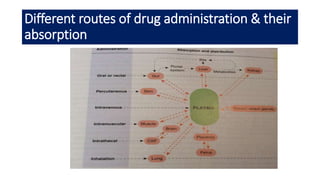
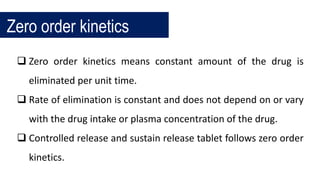
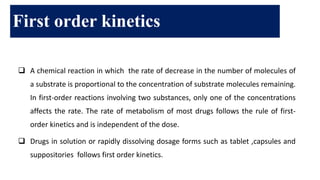
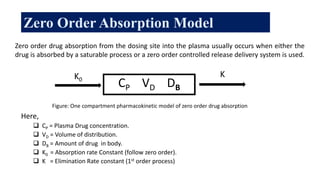
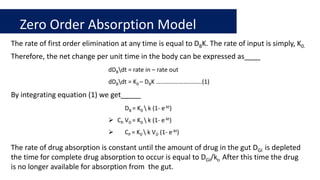
The document discusses pharmacokinetics, which describes how the body affects a drug after administration through absorption, distribution, metabolism, and excretion. It covers factors that affect oral drug absorption like physicochemical properties, dosage form characteristics, and gastrointestinal physiology. The document also explains zero-order and first-order kinetic models, describing constant drug absorption and elimination rates over time.






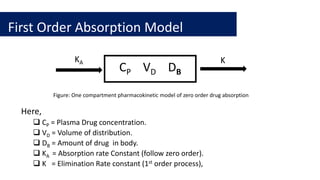
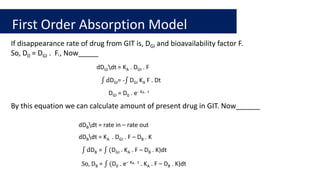
![First order absorption model
=> DB =
D0 .KA. F (e −K . t − e− KA . t)
KA−K
So, CP
=
D0 .KA. F (e −K . t − e− KA . t)
VD (KA−K)
Because,
CP = K0 k VD (1- e-kt)[ ]](https://image.slidesharecdn.com/presentation1-171127020535/85/First-order-absorption-model-17-320.jpg)
