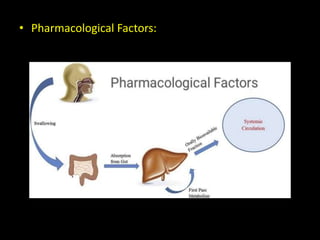
The document discusses the concept of bioavailability and its distinctions from bioequivalence, chemical equivalence, and therapeutic equivalence. It outlines the pharmaceutical and pharmacological factors that influence bioavailability, such as drug formulation, administration route, and individual patient factors. Additionally, it highlights important variables like drug interactions, food effects, and first-pass metabolism that can significantly impact the absorption and effectiveness of medications.