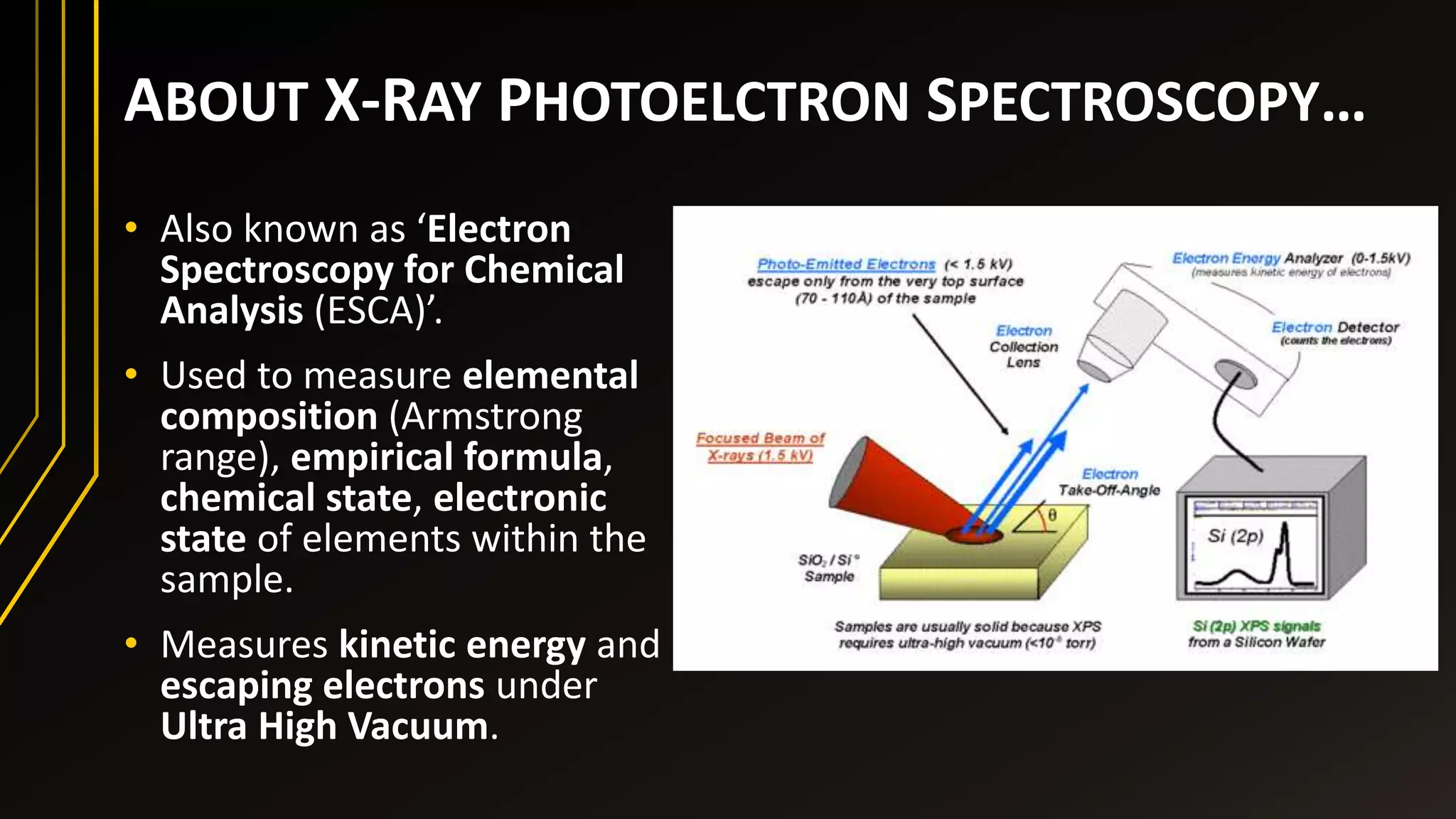
This document discusses X-ray photoelectron spectroscopy (XPS), a technique used to analyze the surface chemistry of materials. XPS uses X-rays to excite photoelectrons from the top 1-10 nm of a sample, and measures their kinetic energy to determine the elemental composition, empirical formula, chemical and electronic states. It works by relating the photoelectrons' binding energy to the energy of the exciting X-ray photons minus the kinetic energy and work function. XPS can identify all elements except hydrogen and helium, and is useful for quantifying elemental composition and chemical bonding at surfaces.