Thermal Energy Relationships Explained
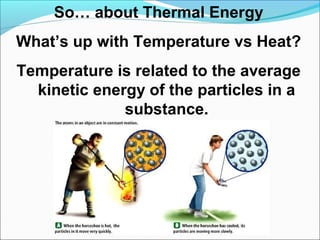
- 1. So… about Thermal Energy What’s up with Temperature vs Heat? Temperature is related to the average kinetic energy of the particles in a substance.
- 2. You know the SI unit for temp is Kelvin K = C + 273 (10C = 283K) C = K – 273 (10K = -263C) Thermal Energy is the total of all the kinetic and potential energy of all the particles in a substance.
- 3. Thermal energy relationships As temperature increases, so does thermal energy (because the kinetic energy of the particles increased). If the temperature stays the same, the thermal energy in a more massive substance is higher (because it is a total measure of energy).
- 4. Heat Cup gets cooler while hand gets warmer The flow of thermal energy from one object to another. Heat always flows from warmer to cooler objects. Ice gets warmer while hand gets cooler
- 5. Specific Heat (c, sometimes s, but usually c) Things heat up or cool down at different rates. Land heats up and cools down faster than water, and aren’t we lucky for that!?
- 6. Specific heat is the amount of heat required to raise the temperature of 1 kg (but in Chem we use g) of a material by one degree (C or K, they’re the same size). C water = 4184 J / kg C (“holds” its heat) C sand = 664 J / kg C (less E to change) This is why land heats up quickly during the day and cools quickly at night and why water takes longer.
- 7. Why does water have such a high specific heat? water metal Water molecules form strong bonds with each other water molecule; (including H-bonds!)so it takes more heat energy to break the bonds. Metals have weak bonds (remember the “sea of e-) and do not need as much energy to break
- 8. WHERE’S THE MATH, MATE?! Q = m x T x Cp Q = change in thermal energy m = mass of substance T = change in temperature (Tf – Ti) Cp = specific heat of substance
- 9. Specific Heat Capacity If 25.0 g of Al cool from 310 oC to 37 oC, how many joules of heat energy are lost by the Al? heat gain/lose = q = (c)(mass)(∆T) where ∆T = Tfinal - Tinitial q = (0.897 J/g•K)(25.0 g)(37 - 310)K q = - 6120 J Notice that the negative sign on q signals heat “lost by” or transferred OUT of Al.
- 10. Heat can be Transferred even if there is No Change in State q transferred = (c)(mass)(∆T)
- 11. Or… Heat Transfer can cause a Change of State Changes of state involve energy (at constant T) Ice + 333 J/g (heat of fusion) -----> Liquid water Is there an equation? Of course! q = (heat of fusion)(mass)
- 12. Heat Transfer and Changes of State Liquid (l) Vapor (g) Requires energy (heat). Why do you… cool down after swimming ??? use water to put out a fire???
- 13. Remember this – it’s the Heating/Cooling Curve for Water! Woa! Evaporate water Note that T is constant as a phase changes
- 14. So, let’s look at this equation again… q = (heat of fusion)(mass) (There’s also q = (heat of vaporization)(mass), by the way, for when we are talking about vaporization) WHY DO I NEED THIS WHEN I HAVE q transferred = (c)(mass)(∆T) HUH??? Well, when a phase changes THERE IS NO change in temperature… but there is definitely a change in energy!
- 15. So… if I want the total heat to take ice and turn it to steam I need 3 steps… 1) To melt the ice I need to multiply the heat of fusion with the mass…q = (heat of fusion) (mass)
- 16. 2) Then, there is moving the temperature from 0 C to 100C… for this there is a change in temperature so we can use… q transferred = (c)(mass) (∆T) 3) But wait, that just takes us to 100 C, what about vaporizing the molecules? Well, then we need q = (heat of vaporization)(mass)…
- 17. Add ‘em all up and there it is! Now, lucky for us, just like there are tables for specific heats, there are also tables for heats of fusion and heats of vaporization. Whew, At least we don’t have to worry about that!
- 19. Heat & Changes of State What quantity of heat is required to melt 500. g of ice and heat the water to steam at 100 oC? Heat of fusion of ice = 333 J/g Specific heat of water = 4.2 J/g•K Heat of vaporization = 2260 J/g +2260 J/g +333 J/g
- 20. And now… More! Heat & Changes of State How much heat is required to melt 500. g of ice and heat the water to steam at 100 oC? 1. To melt ice q = (500. g)(333 J/g) = 1.67 x 105 J 2. To raise water from 0 oC to 100 oC q = (500. g)(4.2 J/g•K)(100 - 0)K = 2.1 x 105 J 3. To evaporate water at 100 oC q = (500. g)(2260 J/g) = 1.13 x 106 J 4. Total heat energy = 1.51 x 106 J = 1510 kJ
- 21. CALORIMETRY Aka… How we Measure Heats of Reaction In a Constant Volume or “Bomb” Calorimeter, we burn a combustible sample. From the heat change , we measure heat evolved in a reaction to get ∆E for reaction.
- 22. BOOM! Combustible material ignited at constant volume! This heats up the “bomb”, which heats up the water surrounding it… First, some heat from reaction warms the water, which we know the mass of and “c” for… qwater = (c)(water mass)(∆T) THEN, some heat from reaction warms “bomb,” which has a known specific heat for the entire apparatus (typically), so we don’t need the mass… qbomb = (heat capacity, J/K)(∆T) Total heat evolved = qtotal = qwater + qbomb
- 23. The Mathy bit… Measuring Heats of Reaction using… CALORIMETRY Calculate heat of combustion of octane. C8H18 + 25/2 O2 --> 8 CO2 + 9 H2O You could burn 1.00 g of octane… or you could just note that… Temp rises from 25.00 to 33.20 oC Calorimeter contains 1200 g water Heat capacity of bomb = 837 J/K
- 24. VIOLA! Step 1 Calc. heat transferred from reaction to water. q = (4.184 J/g•K)(1200 g)(8.20 K) = 41,170 J Step 2 Calc. heat transferred from reaction to bomb. q = (bomb heat capacity)(∆T) = (837 J/K)(8.20 K) = 6860 J Step 3 Total heat evolved 41,170 J + 6860 J = 48,030 J Heat of combustion of 1.00 g of octane = 48.0 kJ
- 25. One More (ok, LAST) Example… With a twist… If I burn 0.315 moles of hexane (C6H14) in a bomb calorimeter containing 5.65 liters of water, what’s the molar heat of combustion of hexane is the water temperature rises 55.40 C? The heat capacity of water is 4.184 J/g0C. ∆H = mCp∆T ∆H = (5,650 grams H2O)(4.184 J/g0C)(55.40 C) ∆H = 1310 kJ Do you think we’re done?
- 26. NOPE! We were asked for the MOLAR heat of combustion! Tricky, tricky… Molar Heat of Combustion The amount of energy released in burning completely one mole of substance. Vs. Heat of combustion The amount of heat released per unit mass or unit volume of a substance when the substance is completely burned. We also have heat of fusion vs MOLAR heat of fusion and heat of vaporization vs MOLAR heat of vaporization, so be watchful…
- 27. What we calculated is the amount of energy generated when 0.315 moles of hexane is burned, which is close but not quite what we were asked for... To find the molar heat of combustion, we need to multiply this by (1 mole/0.315 moles) = 3.17. As a result, the molar heat of combustion of hexane is 4150 kJ/mol.
Editor's Notes
- {}
