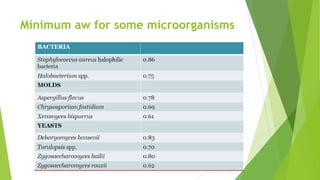
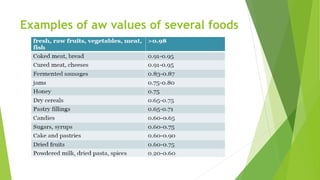
This document discusses water activity, which is a measure of the amount of free water available in a food product for microbial growth. It defines water activity as the ratio of vapor pressure of water in a food to the vapor pressure of pure water at the same temperature. Water activity, not water content, determines the lower limit for microbial growth. Most bacteria do not grow below a water activity of 0.91 and molds below 0.80. Measuring water activity allows prediction of spoilage and is important for controlling microbial growth, chemical reactions, and other quality factors in foods.