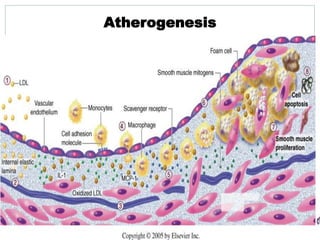
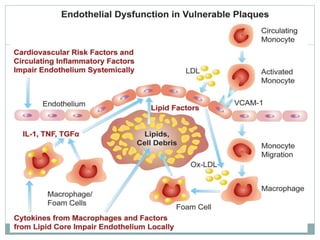
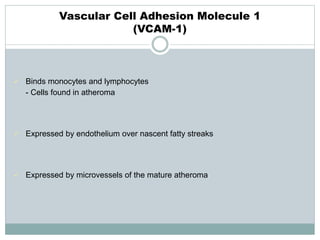
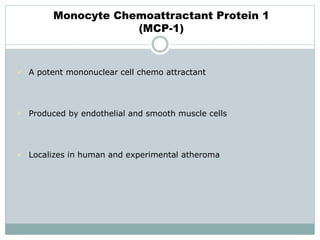
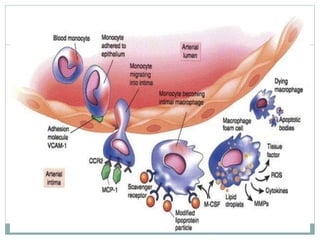
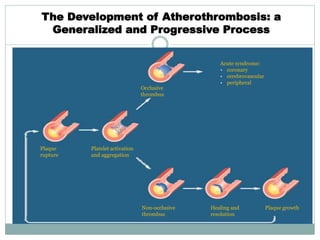
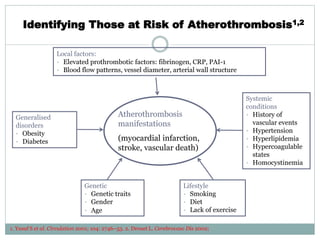
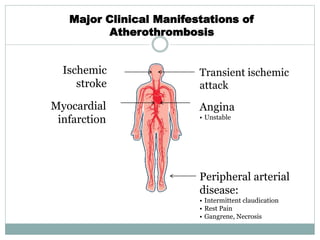
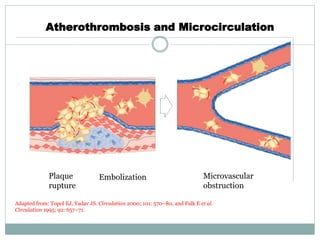
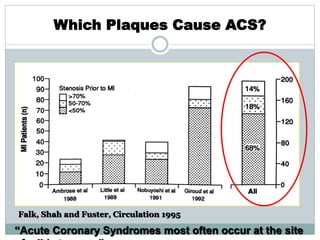
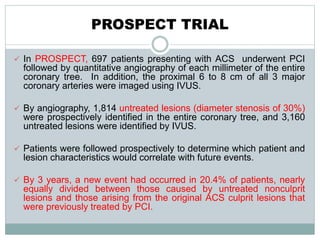
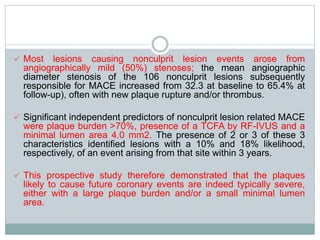
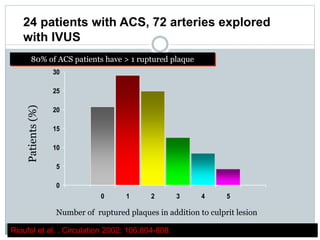
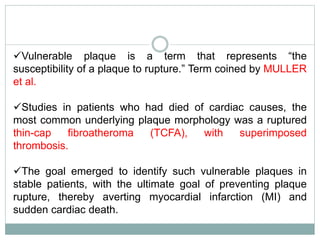
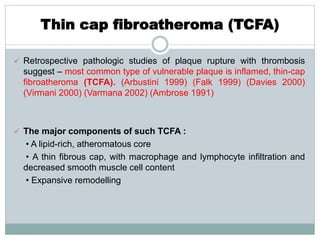
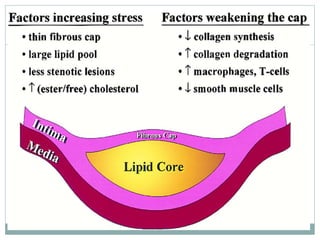
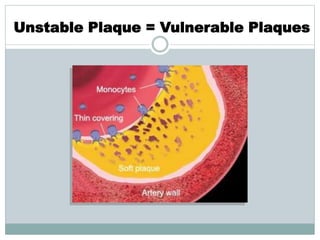
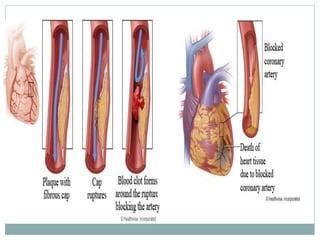
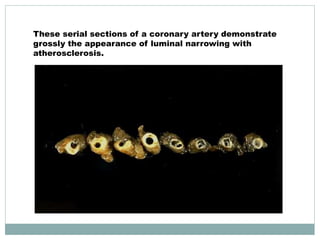
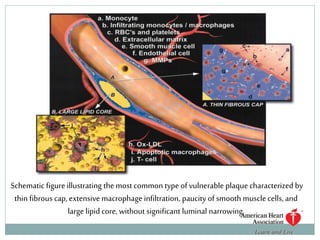
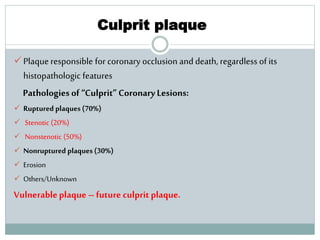
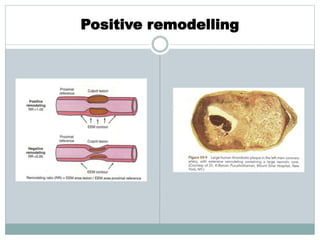
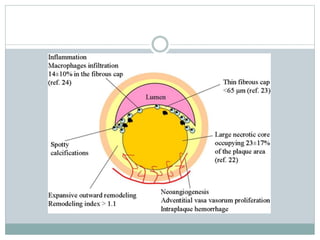
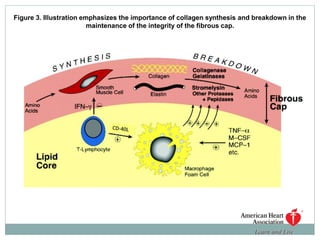
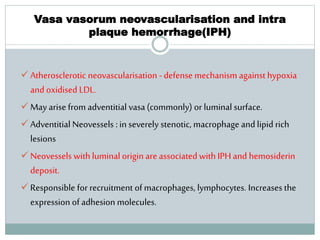
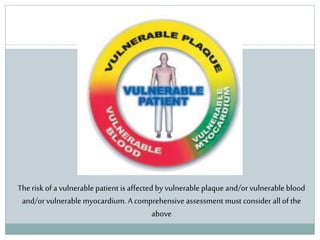
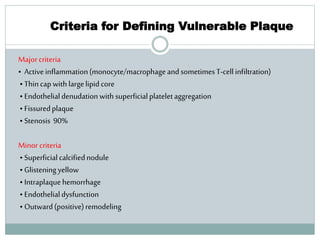
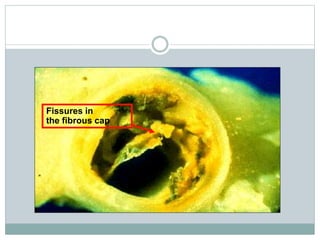
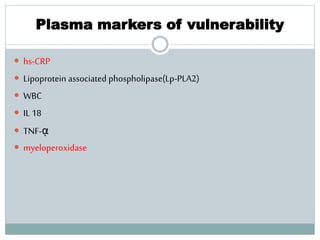
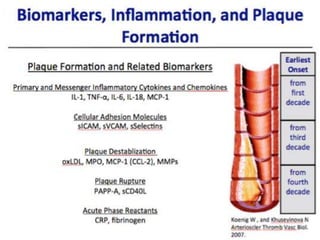
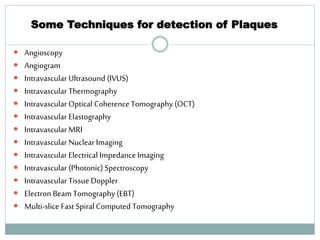
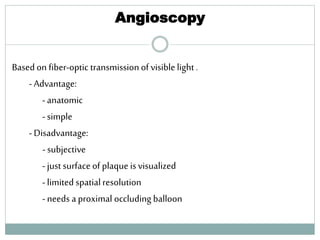
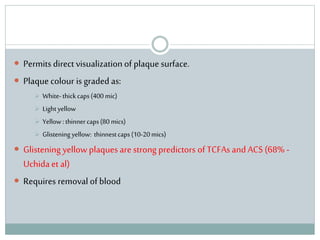
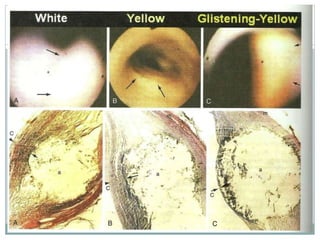
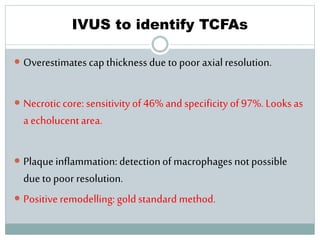
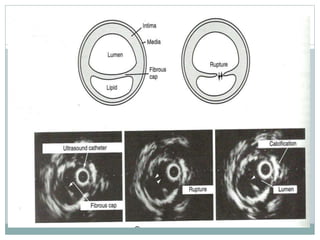
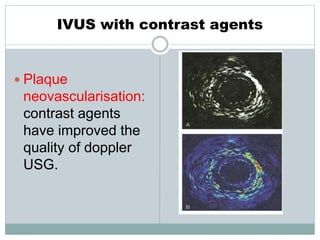
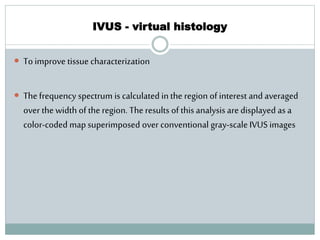
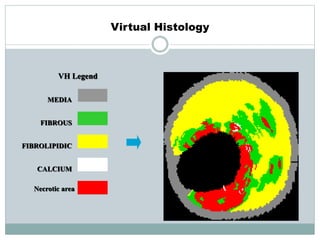
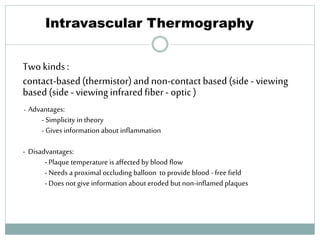
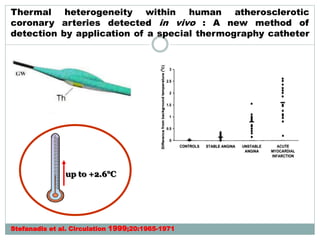
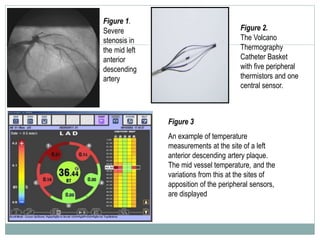
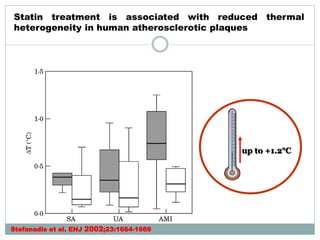
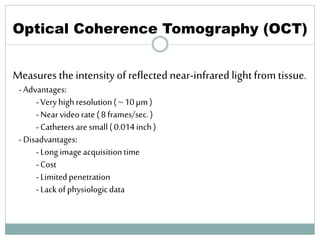
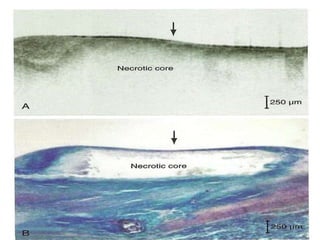
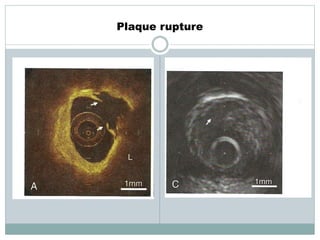
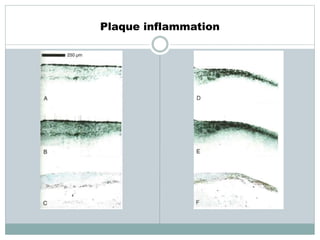
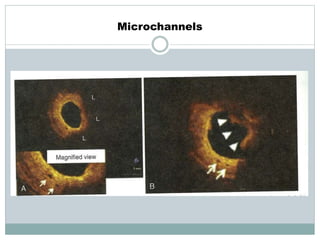
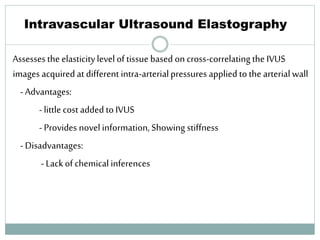
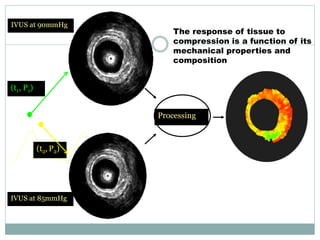
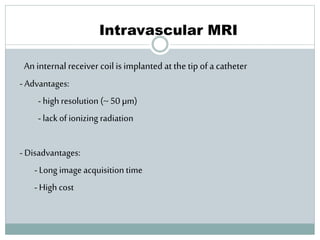
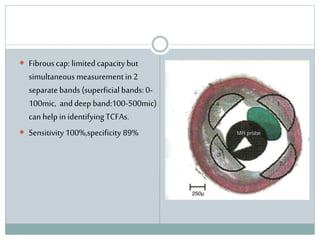
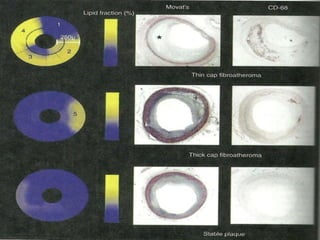
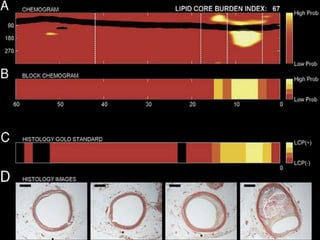
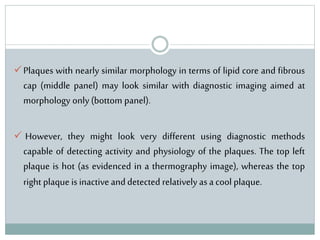
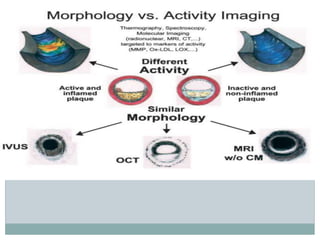
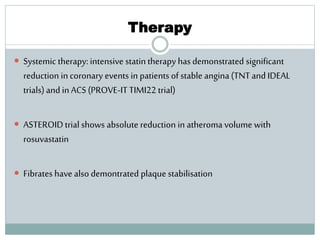
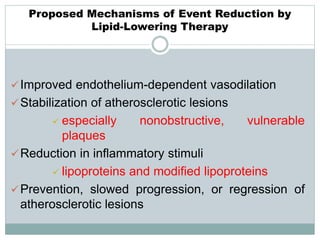
The document discusses vulnerable plaques and patients. It defines vulnerable plaque as a plaque susceptible to rupture. Research has shown that ruptured thin-cap fibroatheromas (TCFAs) are the most common underlying plaque morphology in cardiac deaths. The goal is to identify vulnerable plaques in stable patients to prevent events like heart attack and sudden cardiac death. A vulnerable plaque is typically characterized by a thin fibrous cap, large lipid core, and inflammation. Identifying high-risk features in plaques can help determine which patients are vulnerable.