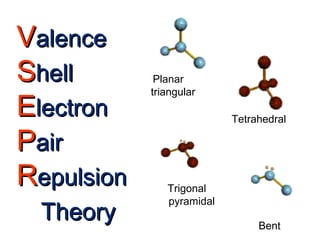
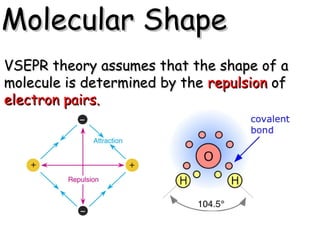
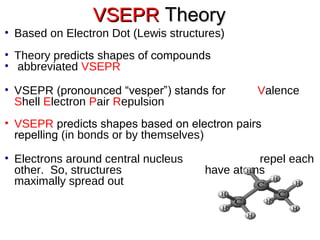
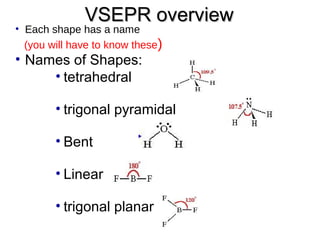
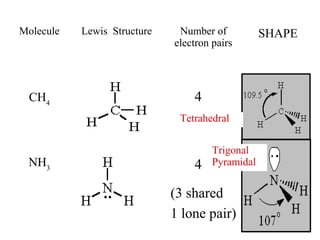
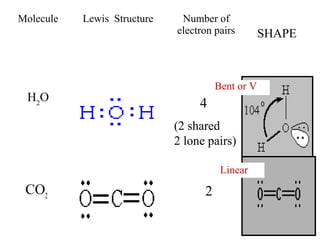
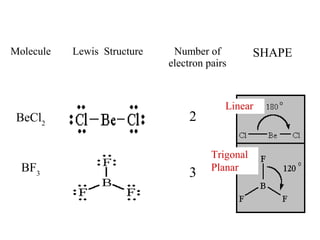
VSEPR theory predicts molecular shapes based on electron pair repulsion around a central atom. It assumes that the molecular structure forms in a way to minimize repulsion between bonding and non-bonding electron pairs. The theory names molecular shapes such as tetrahedral, trigonal pyramidal, bent, linear, and trigonal planar based on the number of electron pairs around the central atom. Examples are given of the Lewis structures and predicted shapes of methane, ammonia, water, carbon dioxide, beryllium chloride, and boron trifluoride.