1. Redox reactions involve both oxidation and reduction processes occurring simultaneously, such as a displacement reaction where one element replaces another in a compound.
2. Metals can be ranked based on their ability to displace hydrogen from sources like water or acids in an activity series. More reactive metals displace hydrogen from less extreme sources.
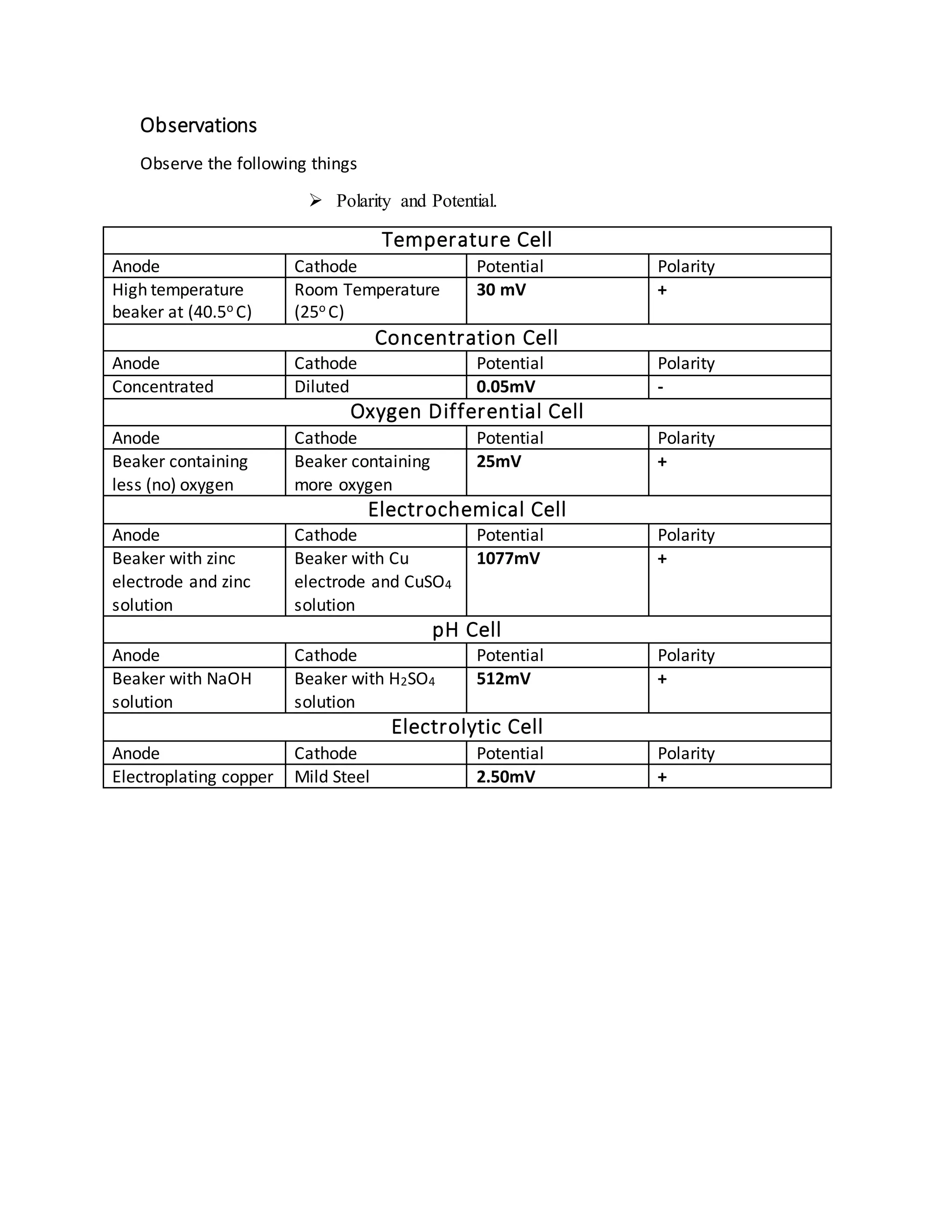
3. Electrochemical cells consist of an anode where oxidation occurs, a cathode where reduction occurs, and an electrolyte for ion transport between electrodes. Different types of cells generate voltage based on factors like temperature, concentration, oxygen levels, or pH.