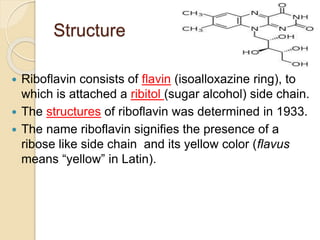
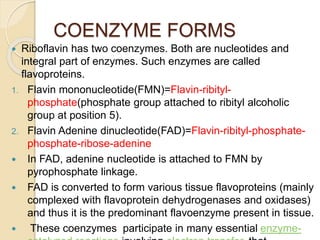
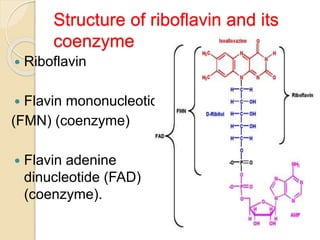
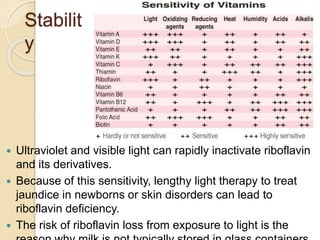
Riboflavin, or vitamin B2, is a water-soluble compound first isolated in 1872, essential for various metabolic processes and found primarily in animal-derived foods. It exists mainly in two coenzyme forms: flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which play critical roles in enzyme function and energy metabolism. Deficiency can lead to symptoms such as cheilosis and glossitis, but toxicity from high intakes is rare; its primary storage site is the liver.