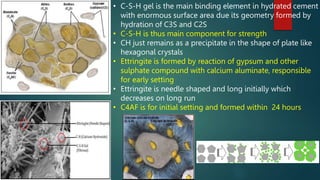
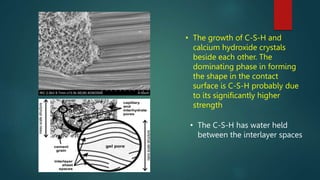
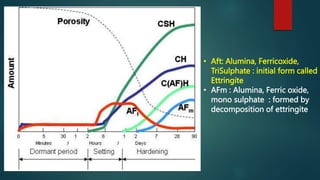
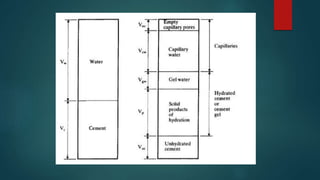
Concrete is a three-phase material consisting of aggregate, hydrated cement paste, and a transition zone between the aggregate and cement paste. The aggregate phase provides strength, weight, and thermal properties to the concrete. The hydrated cement paste phase is made up of products like calcium silicate hydrate and calcium hydroxide and binds the aggregate together. The transition zone between the aggregate and cement paste is weaker due to microcracking and limits the strength of the concrete.