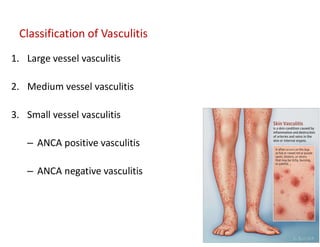
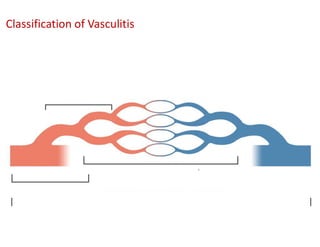
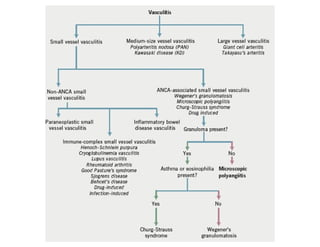
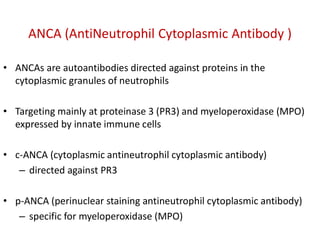
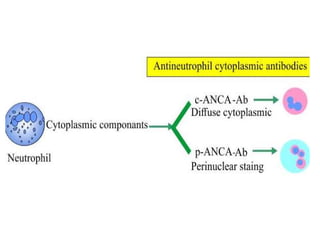
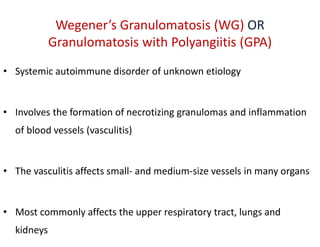
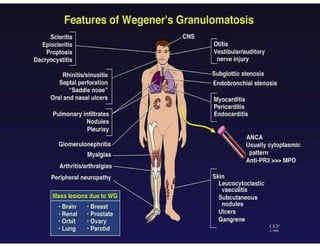
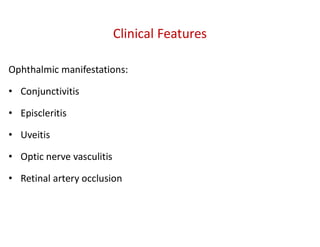
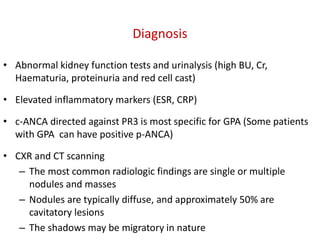
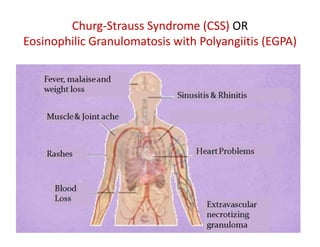
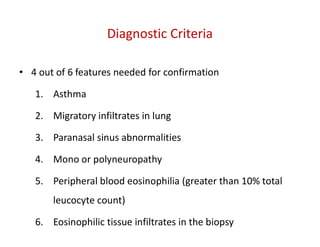
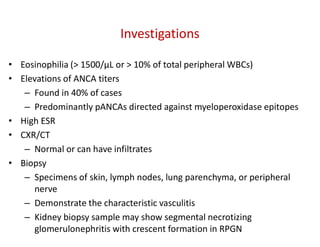
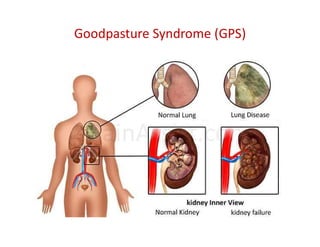
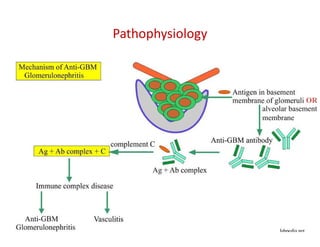
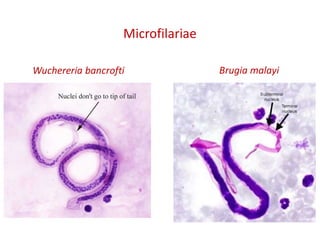
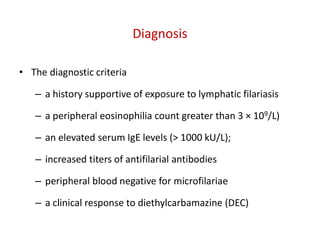
The document discusses various types of vasculitis affecting the lungs, including large, medium, and small vessel vasculitis, detailing classification, clinical features, diagnosis, and management strategies. It emphasizes autoimmune conditions like Granulomatosis with Polyangiitis, Churg-Strauss Syndrome, Anti-GBM disease, and other related pulmonary eosinophilia syndromes. Key clinical manifestations include respiratory issues, renal failure, and systemic symptoms, along with treatment options ranging from immunosuppression to plasmapheresis.