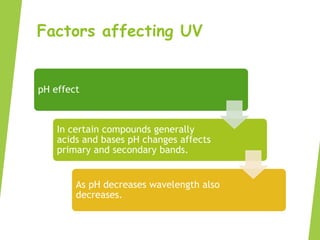
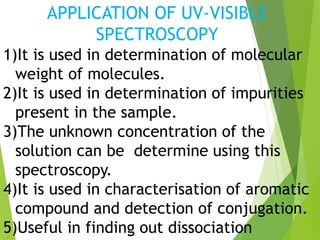
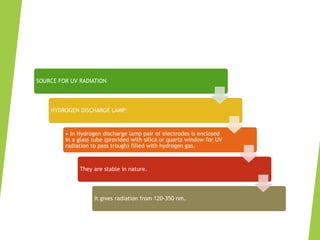
This document provides an overview of UV-visible spectroscopy. It discusses the electromagnetic spectrum and how UV-visible spectroscopy involves absorption of UV or visible light by molecules, promoting electrons to excited states. Beer-Lambert's law describes the relationship between light absorption and analyte concentration. Chromophores and auxochromes determine peak absorption wavelengths. Factors like pH and solvents affect absorption spectra. UV-visible spectroscopy has applications in determining molecular structures, concentrations, and impurities. Instrumentation includes sources like hydrogen lamps, monochromators, sample holders, detectors like photomultiplier tubes, and single or double beam spectrophotometers.