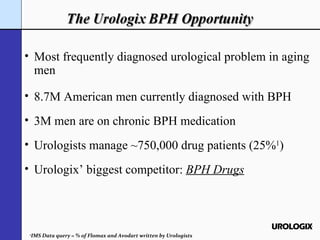
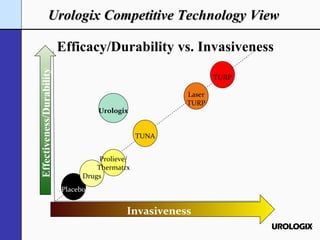
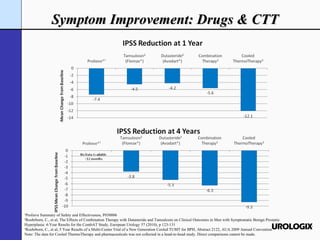
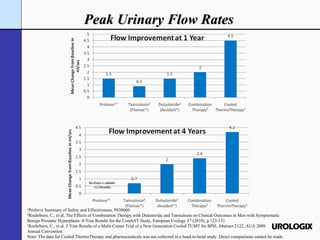
Urologix, Inc. is a medical device company focused on developing and marketing minimally invasive cooled thermotherapy technology for treating benign prostatic hyperplasia (BPH). The presentation outlines Urologix's proprietary technology, market opportunity, and competitive advantages over traditional BPH drug treatments, emphasizing the safety, efficacy, and durability of their solution. The company aims to expand its market presence by converting urologists to their therapy and entering the BPH drug market to enhance patient access.














![Stryker Warren, Jr. Chief Executive Officer 763.475.7605 [email_address] Brian Smrdel Chief Financial Officer 763.475.7696 [email_address] Contacts: Greg Fluet EVP & Chief Operating Officer 763.475.7690 [email_address]](https://image.slidesharecdn.com/urologixonemedforumpresentation63010v2-12789663580406-phpapp01/85/Urologix-Inc-ULGX-26-320.jpg)