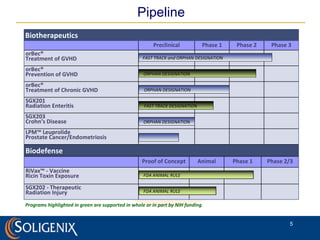
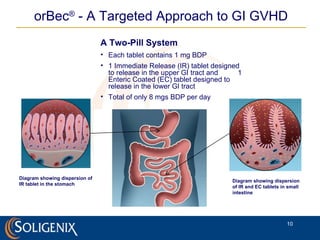
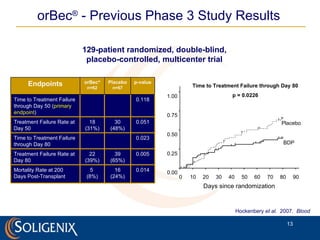
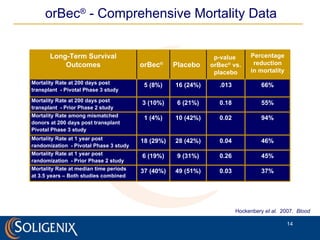
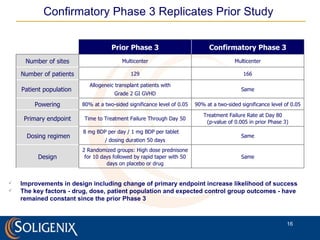
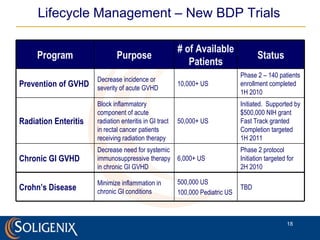
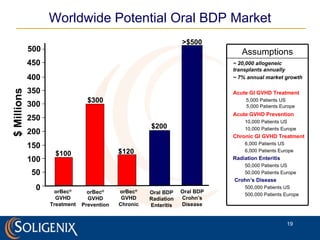
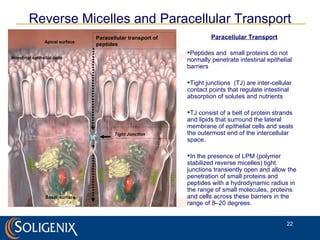
Soligenix, Inc. provides forward-looking statements about its potential growth and development of biopharmaceutical products, particularly focusing on its lead product, orbec®, for gastrointestinal graft-versus-host disease (GVHD). The document outlines various risks related to product development, regulatory approvals, and market competition, while highlighting the company's strong clinical trial data and strategic partnerships for commercialization. Additionally, it discusses other programs in their pipeline, including vaccines for bioterrorism and advanced drug delivery systems.